Adipose Tissue As An Endocrine-Immune Organ In HIV-Associated Lipodystrophy
Antiretroviral therapy’s advent as AIDS treatment in the ‘90s showed increased survival rates of HIV-infected patients. What role does adipose tissue as an endocrine-immune organ play here?
Author:Suleman ShahReviewer:Han JuSep 09, 202412.5K Shares545.7K Views
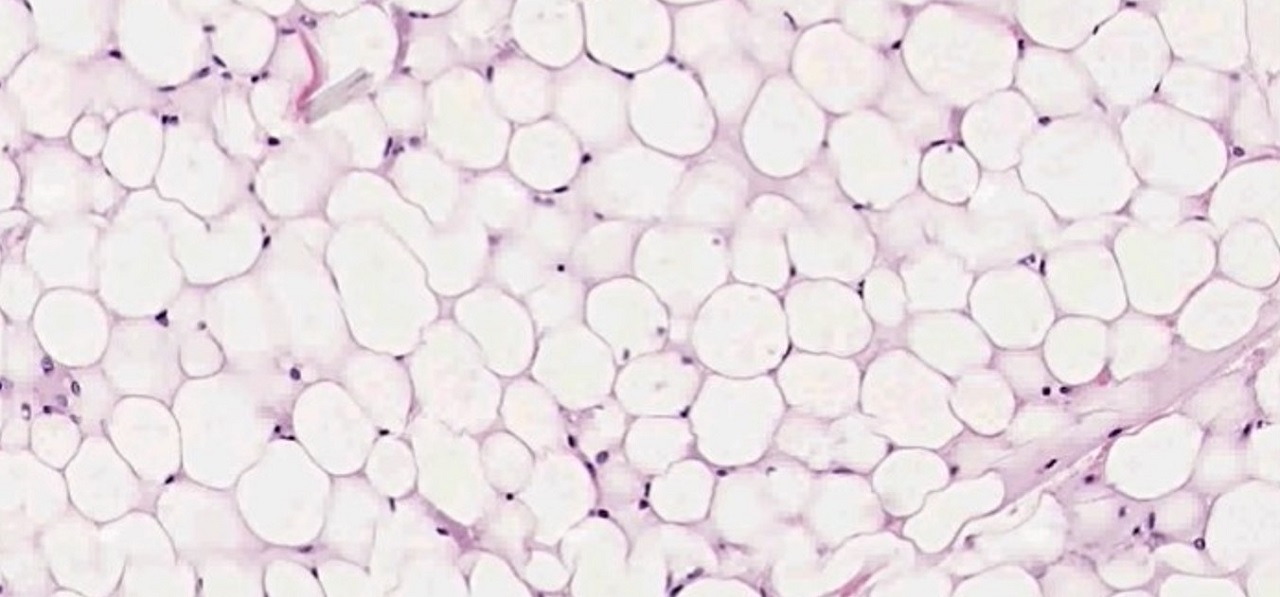
This critical review aims to discuss the mechanisms underlying lipodystrophy and their effects, focusing on adipose tissue as an endocrine-immune organ.
Back in the 1990s, when the medication regimen called highly-active antiretroviral therapy (HAART)was first introduced to treat acquired immunodeficiency syndrome (AIDS), the number of patients infected with human immunodeficiency virus (HIV) who survived it increased. In addition, the quality of their lives improved as well.
Thus, making HIV infection a chronic disease, which leads to an increased prevalence of other pathologies, such as HIV-associated lipodystrophy syndrome (HIVLS).
HIVLS is a clinical picture which consists of changes in body fat distribution and endocrine-metabolic changes.
In HIVLS, there can be:
a. Central lipohypertrophy
The accumulation of fat can occur in the following areas:
- torso and/or abdomen
- breasts
- posterior cervical region
b. Peripheral lipoatrophy
The thinning of adipose tissue may occur in the:
- face
- anterior and lateral cervical
- lower and/or upper limbs or buttocks
c. Mixed lipodystrophy
This happens when both alterations can occur.
These changes can take place under different intensities.
This syndrome can present, associated with changes in body fat distribution, other metabolic features, such as:
- dyslipidemia
- insulin resistance
Physiopathology Of HIVLS - Lipoatrophy And Lipohypertrophy
Several risk factors can be associated with lipodystrophy.
The risk factors associated to greater risk of lipohypertrophy are:
- the use of antiretroviral therapy, duration of antiretroviral therapy
- the use of protease inhibitors
- female gender
- greater amount of body fat before antiretroviral therapy
- undetectable viral load
As to lipoatrophy, the risk factors are:
- age (patients over 40 years old)
- low body fat prior to antiretroviral therapy
- lower levels of CD4+ T cells
- the use of stavudine
- duration of antiretroviral therapy treatment
- HIV infection time
Regardless of the clinical form, studies indicated the following as risk factors:
- the use of HAART
- age
- dyslipidemia
- pre-infection
- CD4+ T cells values
There are distinct physiopathological mechanismsof lipodystrophy, which is the reason why there is greater knowledge regarding lipoatrophy.
Among the physiopathological mechanisms, mitochondrial toxicity is one of the most important.
In addition to the impaired expression of genes associated with adipogenesis, the adipose tissue in patients with HIVLS, especially those who receive nucleoside analogues (NRTIs) show a reduction in the expression of mitochondrial DNA (mtDNA).
It suggests that the depletion of mtDNA, which is mediated by NRTI, plays a role in lipodystrophy’s pathogenesis.
In spite of that, a simple explanation to NRTIs action based on the reduction of oxidative burst is not enough to adipocyte differentiation and increased lipolysis may be involved.
It was reported that the following caused a decrease in lipid content, mitochondrial activity, and survival of adipocytes in vitro:
- thymidine analogues
- stavudine
- zidovudine
Protease inhibitors can also cause lipoatrophy by:
- inhibiting lipogenesis and adipocyte differentiation
- stimulating lipolysis
- preventing the nuclear localization of sterol regulatory enhancer-binding protein 1 (SREBP1).
Although the consequences of mtDNA depletion are not completely comprehended, it is known that damages to mitochondrial oxidative metabolism can lead to disturbance in gene expressions (adiponectin, for example) through:
- retrograde signaling
- oxidative damages in lipids management
Such disturbance favor:
- the accumulation of fat
- mitochondrial lipotoxicity
Thereby, impaired mitochondrial function appears as an additional characteristic to changes in lipoatrophic subcutaneous fat.
Through subcutaneous adipose tissue biopsy of HIV-1 infected patients with lipoatrophy, high levels of expression of inflammatory markers as tumor necrosis factor (TNF-α) were observed (IL-6, IL-8, and IL-18).
The expression of the first two is positively correlated with apoptosis and negatively with the expression of adipogenic markers, considering the proinflammatory role of cytokines in adipocyte differentiation and viability.
Certain similar alterations in inflammatory markers were observed, such as induced TNF-α, while it differs from other subcutaneous adipose tissue markers, such as the deficiency in the induction of monocyte chemoattractant protein-1 (MCP-1).
Still, regarding immunohistochemistry, the expression of tumor growth factor beta (TGF-β), TNF-α, and caspase 3 were analyzed in the adipose tissue of patients with lipodystrophy.
After the analysis, an enhanced expression of caspase 3 and TNF-α were found, which has a correlation with the expression of TGF-β, suggesting negative feedback between an inflammatory response and an anti-inflammatory response.
The inflammatory response was more important in male patients.
The continued use of HAART, specially of protease inhibitors, leads to alterations of Peroxisome Proliferator-Activated Receptor gamma (PPARγ) of macrophages stimulating their transformation to M1 or inflammatory or classical activated macrophage, which can:
- induce apoptosis
- increase secretion of inflammatory cytokines (e.g., TNF-α and IL-6)
- contribute to mechanisms of lipodystrophy’s clinical expression
The lipohypertrophy-associated mechanisms have not been established yet.
However, lipolysis is more intense in the subcutaneous adipose tissue by the inflammatory cytokines’ activity, such as TNF-α and IL-6. Nevertheless, these effects were not the same as observed in the visceral adipose tissue.
In the attempt to minimize lipotoxic damage to other tissues, increased visceral adiposity is presented as a protective mechanism of the organism due to acquired generalized lipodystrophy (AGL).
HIV-Associated Lypodystrophy Syndrome And Endocrine And Metabolic Alterations
The HAART consists of a specific treatment developed to improve the healthstatus of HIV-1-infected patients.
It consists of a combination of an NRTI, non-nucleoside reverse transcriptase inhibitor (NNRTI), associated with protease inhibitor (PI).
Almost half of HIV-1-infected patients under HAART (40% to 50%) present changes in adipose tissue distribution (known as lipodystrophy) in addition to systemic metabolic complications.
Lipodystrophy is classified into three clinical categories:
- lipohypertrophy
- lipoatrophy
- mixed syndrome
This classification considers the redistribution of body fat in individuals living with HIV.
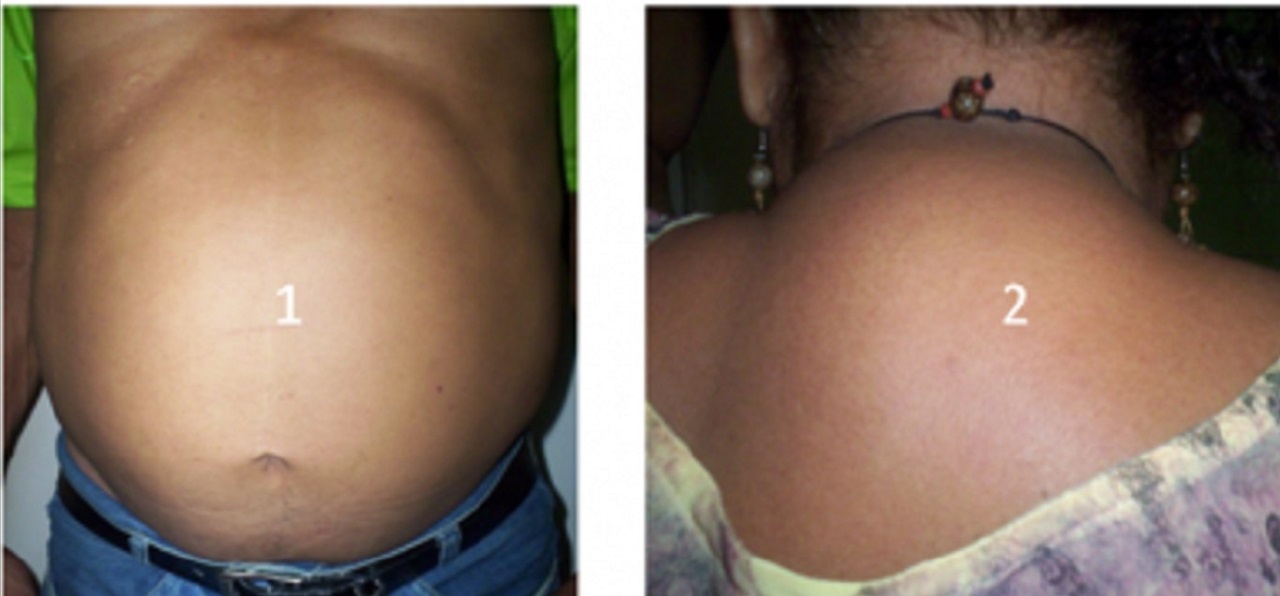
Lipohypertrophy is characterized by accumulation of fat in the:
- abdominal area
- visceral fat accumulation
- dorsal-cervical area (known as buffalo hump)
- breasts
Lipoatrophy occurs when the patient has lost subcutaneous fat in the face, buttocks, and upper and lower limbs, making its blood vessels prominent.
The mixed form refers to a combination of both lipohypertrophy and lipoatrophy.
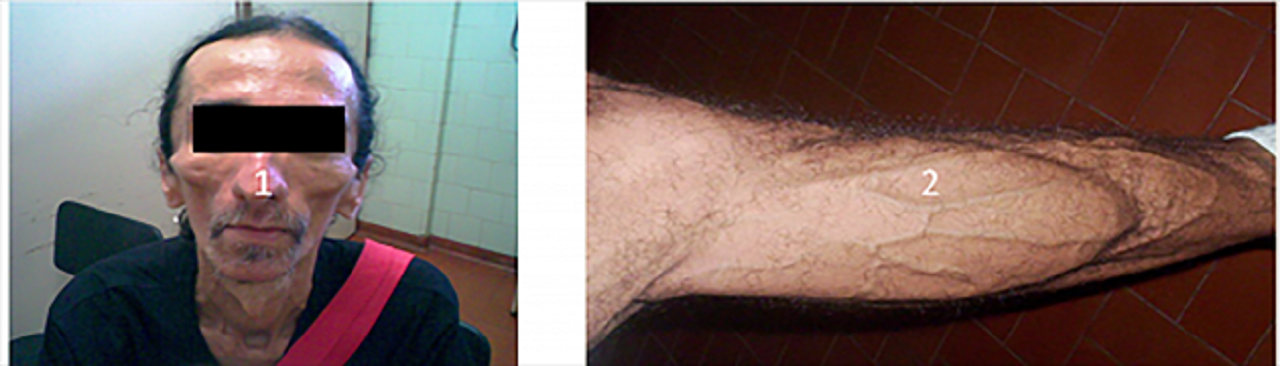
It is necessary to remember that these alterations can reach patients differently.
Besides, this group of patients are more susceptible to some metabolic alterations, such as:
- dyslipidemia
- insulin resistance
- enhanced risk of cardiovascular disease
A frequency of the following was reported:
- 21.1 % of diabetesmellitus
- 48.7% of mixed dyslipidemia
- 32.9% of hypertriglyceridemia
- 10.5% of low HDL (high-density lipoprotein) cholesterol
However, in another study, higher frequencies were observed, such as:
- 61.6 % of hypertriglyceridemia
- 75.8 % of low HDL
It suggests that these findings depend on the study of each individual case.
The association between the clinical signs of lipodystrophy with a specific drug of HAART is not well established yet. It is suggested that NRTIs favor lipoatrophy and PIs tend to promote visceral lipohypertrophy and systemic metabolic disturbances.
Nevertheless, recent studies suggest they act in synergism to generate the characteristic signs of the redistribution of body fat and thus, contribute to associated metabolic alterations.
The adipose tissue, as a secretory organ, releases:
a. Hormones, such as:
- leptin
- adiponectin
b. Cytokines, such as:
- TNF-α
- IL-1 (interleukin 1)
- IL-6 (interleukin 6)
They present an important immune function in inflammatory response, which will reflect in metabolic alterations in lipodystrophy syndrome.
In lipoatrophy, a decrease in the expression of PPARγ occurs, leading to alterations in the lipid metabolism, causing a reduction of lipoprotein lipase.
Lipoprotein lipase is the enzyme responsible for the absorption of fatty acids by the adipose tissue and fat replacement that acts by hydrolysis of circulating triglycerides, which can also justify the hypertriglyceridemia observed in lipodystrophy.
The expression of the insulin-sensitive glucose transporter GLUT4 is also reduced, leading to:
- impaired glucose uptake
- consequent reduction in triglyceride synthesis
Another major alteration that is probably related to the impairment of the PPARγ activity is the reduced expression of adiponectin.
The known role of adiponectin in the improvement of glucose uptake mediated by insulin in peripheral tissues and liver to sensitize insulin-dependent suppression of glucose production causes such reduction to be considered a major contributor to insulin resistance.
In a study with 112 HIV-infected patients treated with highly-active antiretroviral therapy (HAART), higher levels of adiponectin in patients without lipodystrophy were observed compared to individuals that showed body fat redistribution.
This study suggests that in HIV-infected patients treated with HAART, adiponectin would be:
a. inversely correlated with:
- visceral fat mass
- triglycerides
- insulin resistance
b. directly associated to:
- HDL cholesterol
- peripheral fat
As to leptin, it is reduced in patients with lipoatrophy.
Low levels of leptin were independently associated with insulin resistance in patients with:
- lipoatrophy
- posteriorly to peripheral and total body fat adjustment
This has to do, probably, with the reduction of total body fat and consequent reduction in leptin production by adipocytes.
Individuals with lipohypertrophy showed the highest serum leptin levels, probably due to:
- a state of leptin resistance; or
- excessive production of leptin by adipose tissue
In patients with lipohypertrophy, free fatty acids from lipolysis in visceral fat, released in great quantities in portal circulation, play an important role in the genesis of tissue resistance to insulin action, both hepatic and peripheral levels.
Two major things were noticed in women with HIV-associated lipodystrophy, which is similar to metabolic syndrome:
a. increase in:
- waist-hip ratio
- central adiposity
- triglycerides
- LDL (low-density lipoprotein) cholesterol
b. decrease in HDL (high-density lipoprotein) cholesterol
In addition to the cardiovascular risk factors, C-reactive protein and IL-6 were elevated and adiponectin was reduced.
It is interesting, as observed, that pro-inflammatory cytokine such as TNF-α is elevated in patients with HIV-associated lipodystrophy, and it is an important factor in insulin resistance.
Therefore, both lipoatrophy and lipohypertrophy are alterations of the adipose tissue that can lead to insulin resistance and dyslipidemia, with major underlying inflammatory response.
Conclusion
HIV-infected patients, in chronic use of HAART, commonly develop the lypodystrophy syndrome and have aggressiontowards the adipose tissue, its main form of attack, even though its genesis remains not completely clarified.
Among them, we mention mitochondrial toxicity.
Specifically, we highlighted on the alterations of the adipose tissue:
- when it comes to endocrine functions (e.g., adiponectin and leptin)
- when it comes to immune functions (e.g., interleukins)
- when it involves body distribution, resulting in clinical evidence of seemingly peripheral lipoatrophy and/or central lipohypertrophy
Highlighting these alterations resulted in clinical evidence of seemingly peripheral lipoatrophy and/or central lipohypertrophy.
The HIV lypodystrophy syndrome enables endocrine-metabolic changes, such as insulin resistance and alterations in glucose tolerance profile, with a tendency to evolve into diabetes.
In addition, in some specific cases, the syndrome also enables non-alcoholic hepatic steatosis.
Dyslipidemic alterations are present in the majority of patients, which makes it easier for the prevalence of the metabolic syndrome.
Therefore, taking into consideration the manifestations of the syndrome, these patients hold a high-risk endocrine-metabolic profile for cardiovascular events.
Due to this fact, it becomes necessary to research more about adipose tissue as an endocrine-immune organ and to look for strategies that aim for the prevention and control of possible comorbidities to enhance the quality of lifeof HIV-infected patients.

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles