Emerging Roles Of Hematopoietic Lineage Cell-Specific Protein 1 In The Immune System
HS1 or hematopoietic lineage cell-specific protein 1 is associated with various diseases. More research will continue to uncover its role in different cellular processes.
Author:Suleman ShahReviewer:Han JuDec 30, 20234.2K Shares281.8K Views
Also known as HCLS1, hematopoietic lineage cell-specific protein 1(HS1) is a protein that plays a role in the regulation of the immune systemand hematopoiesis (the formation of blood cells).
HS1 was initially characterized as a substrate of kinases coupled to T-cell and B-cell receptors, such as these two members of the Src family of protein tyrosine kinases (SFK):
- Lck (lymphocyte-specific protein tyrosine kinase)
- Lyn (Lck/Yes-related novel protein tyrosine kinase)
HS1 is a multidomain protein that activates the Arp2/3 complex to promote the polymerization of branched actin.
It is the immune system paralogue of cortactin, a ubiquitous protein implicated in cell migration, endocytosis and invasion of host cells by many bacterial pathogens.
HS1 in some ways substitutes for cortactin in the immune system.
For example, it participates in cell migration by helping assemble specific actin structures, such as podosomes in dendritic cells.
At the same time, HS1 plays several roles in the immune system that its cortactin paralogue does not.
Not only does HS1 act downstream of T-cell and B-cell receptors to promote actin polymerization but it also acts downstream of the high-affinity IgE receptor (FcERI) in mast cells and it assists natural killer cells in forming the lytic immunological synapse.
Since its discovery in the late 1980s, HS1 has gradually been implicated in diverse pathological conditions of the immune system, including:
- leukemia
- lupus
- severe congenital neutropenia
The present review summarizes the current knowledge of HS1 regulation and participation in molecular complexes, focusing on key aspects of HS1 in immunological disease.
Preliminary Discussion
In 1989, researchers described a new human protein expressed in lymphoid, myeloid, and erythroblastoid cell lines, as well as in peripheral blood lymphocytes, granulocytes, and macrophages.
However, it was not detected in non-hematopoietic tissues and so was named hematopoietic lineage cell-specific protein 1 (HS1).
Soon afterwards, its gene was mapped to human chromosome 3q13, and it was identified as a substrate of Lyn kinase.
As a result, HS1 is sometimes referred to as hematopoietic cell-specific Lyn substrate 1 (HCLS1).
The ubiquitous paralogue of HS1 is cortactin, encoded by the CTTN gene (formerly EMS1), which is located in a chromosomal region frequently amplified in human carcinomas.
Like cortactin, HS1 promotes actin polymerization by activating the Arp2/3 complex.
In this way, it participates in multiple processes that remodel the actin cytoskeleton, including:
- immunological synapse formation
- cell adhesion
- cell migration
Cortactin was initially described as a cytoskeletal protein and a major substrate of Src kinase in studies conducted in chicken embryonic fibroblasts.
Therefore, investigators have assumed that HS1 and cortactin show mutually exclusive expression patterns.
However, this has never been tested in depth.
Such expression analysis is particularly important for immune system studies, since HS1 is generally assumed to play the role of cortactin in immune cells.
Although pathogens exploit cortactin for adhesion, invasion and immune avoidance, no evidence yet exists that pathogens take advantage of HS1 in a similar manner.
Clearly, fundamental aspects of HS1 expression and regulation remain unknown, and this poses a problem for gaining deeper insights into immune function.
The aim of this review is focused on the emerging roles of HS1 in the immune system.
Domain Organization Of HS1
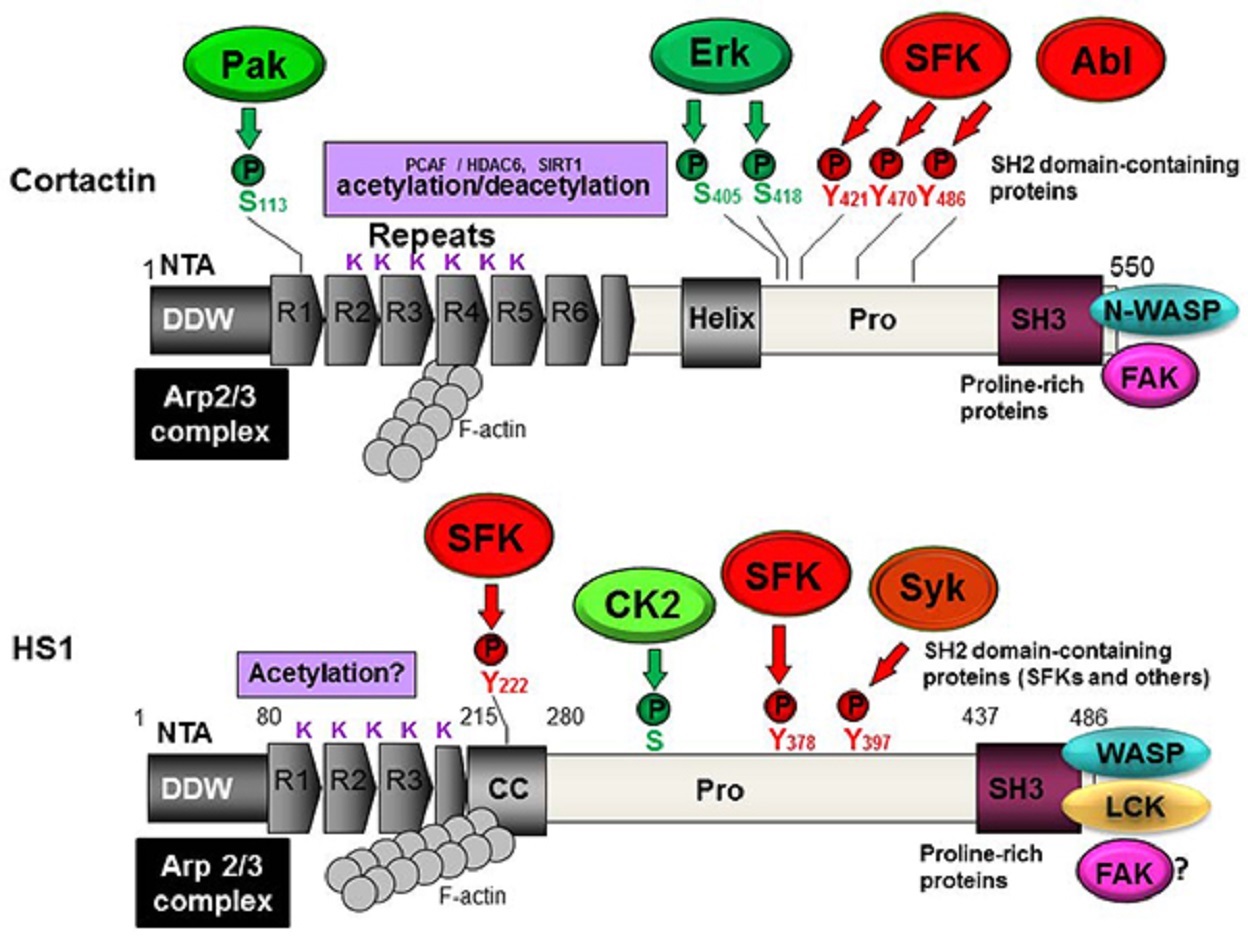
HS1 is a multidomain protein that participates in several cytoskeleton rearrangement processes.
HS1 has an N-terminal acidic (NTA) domain that directly binds and activates the Arp2/3 complex; in this way, HS1 behaves as a nucleation-promoting factor (NPF).
According to the current model for actin polymerization, globular monomeric actin (G-actin) assembles into filamentous actin (F-actin).
The dimeric or trimeric nucleus that initially forms is unstable; so, further polymerization is promoted and controlled by facilitator proteins.
One such protein is the Arp2/3 complex, a seven-membered complex comprising actin-related proteins 2 and 3, as well as five other subunits.
This complex is able to add a “branch” to the side of a pre-existing actin filament, giving rise to branched filaments with a characteristic 70-degree angle.
Cortactin and HS1 appear to bind and activate the Arp2/3 complex in similar ways. So, they are usually considered members of the same NPF (nucleation-promoting factor) family.
The NTA domain of HS1 is followed by three and a half tandem repeats of amino acids, followed in turn by a coiled-coil (CC) region.
The repeats and CC region bind synergistically to F-actin.
Cortactin, in contrast, has six and a half repeats, the fourth of which is required to bind to F-actin and contribute to cortactin activity.
The C-terminus of HS1 contains an SH3 domain highly homologous to the one in cortactin.
Numerous other proteins also act as NPFs, but they activate Arp2/3 by a different mechanism from that of cortactin/HS1.
Wiskott-Aldrich syndrome proteins (WASPs) are an important example.
The canonical member of the family, named WASP, is expressed exclusively in the immune system. Mutation of its gene leads to an immune deficiency called WAS.
A ubiquitous member of the family is neural WASP (N-WASP).
WASP and N-WASP are co-expressed in some cell types, such as:
- macrophages
- dendritic cells (DCs)
These proteins make up a special class of NPFs called class I NPFs. They bind G-actin and activate the Arp2/3 complex through an acidic VCA domain.
In recent years, many other class I NPFs have been described, including WAVE/Scar-related proteins.
In contrast to class I NPFs, HS1 and cortactin are considered “weak” activators of the Arp2/3 complex in vitro, which raises the question of whether they must undergo post-translational modifications or form complexes with other proteins in order to become fully active.
It is also possible that they influence actin remodeling indirectly, by acting through another NPF.
In support of this idea, cortactin can indirectly promote actin nucleation by activating N-WASP in vitro.
Whether HS1 can activate WASP in a similar fashion is unknown.
HS1 Tyrosine Phosphorylation
HS1 contains 486 amino acids and has a predicted molecular weight of approximately 54 kilodalton (kDa). However, it migrates as a single major band of 75 kDa on SDS-PAGE (sodium dodecyl-sulfate polyacrylamide gel electrophoresis).
This strongly suggests the presence of post-translational modifications, which may regulate HS1 activity.
Indeed, studies have shown that several tyrosines in HS1 are phosphorylated by non-receptor-type tyrosine kinases of the Src family.
The major Src family kinases (SFKs) are:
| c-Src | Lyn |
| c-Yes | Hck |
| Fyn | Lck |
| c-Fgr | Blk |
These eight proteins are activated in concert with transmembrane receptors, such as:
- integrins
- growth factor receptors (GFRs)
- immune cell receptors (e.g., T-cell receptor and B-cell receptor)
In this way, SFKs regulate a wide range of cellular processes, including:
- cell growth and differentiation
- survival
- cell adhesion and migration
It appears that HS1 is regulated not only by single phosphorylation events but also by sequential phosphorylation.
Such sequential phosphorylation may be catalyzed by spleen tyrosine kinase (Syk), a member of the Zeta-associated protein of the 70-kDa (Zap70) kinase family, as well as by an SFK acting in concert with other kinases.
The proposed mechanism is that Syk phosphorylates tyrosine 397, creating a docking site for the SH2 domain of SFKs, which then further phosphorylates the protein.
Cortactin was the first member of its NPF family to be described as a substrate of the Abelson (Abl) and Abl-related gene (Arg) non-receptor kinases (Abl/Arg family kinases).
This phosphorylation plays an important role in regulating the actin cytoskeleton.
More recent work has shown that HS1 interacts with Abl kinase during immune synapse formation.
Cortactin is regulated by reversible acetylation of lysines, and the SH3 domain of HS1 binds preferentially to lysine and proline-rich motifs.
This suggests that HS1 may also be structurally regulated by acetylation.
We have recently described a competition between acetylation and tyrosine phosphorylation of cortactin and that phosphorylation inhibits cell spreading.
In addition, work by our group and othershas recently described the participation of cortactin and focal adhesion kinase in regulating focal adhesion turnover.
This suggests that in the near future, HS1 will also be further implicated in integrin signaling.
HS1 Serine/Threonine Phosphorylation
In platelets, casein kinase 2 (CK2) was shown to phosphorylate serines (Ser) and threonines (Thr) in HS1.
Mapping studies showed that phosphorylation occurs primarily at threonines in the N-terminal region and at serines in the central core of the molecule.
Interestingly, Ser/Thr phosphorylation of HS1 potentiates subsequent tyrosine phosphorylation.
HS1 Knockout Mouse Model
HS1 knockout mice show normal development of major lymphoid cell subpopulations; numbers of CD4-positive and CD8-positive T-cells are normal, although negative selection in the thymus is partially impaired.
Numbers of B-cells are also normal, although production of antibodies against T-independent antigens is reduced.
The mice show impaired proliferation of splenic B-cells and T-cells following cross-linking of antigen receptors.
These results indicate that HS1 plays critical roles in antigen receptor-mediated signaling.
More recent studies have identified a profound myeloproliferative block (see Neutropenia). Further studies of this knockout mouse will undoubtedly advance our understanding of HS1 functions.
HS1 And B-Cells
When the B-cell receptor (BCR) binds specific antigen, B-cells respond by:
a. differentiating into plasmocytes (plasma cells), which then generate antibodies
b. generating long-lived memory cells, thereby contributing to a protective adaptive immune response
After cross-linking of membrane-bound IgM, HS1 is tyrosine phosphorylated and associates with the SFK Lyn. In fact, Lyn and Syk kinases have been shown to synergistically phosphorylate HS1, principally on tyrosines 378 and 397.
These findings strongly suggest that HS1 plays important roles during the activation of B cells.
Consistent with this idea, low expression of HS1 correlates with resistance to BCR-induced apoptosis.
Many questions remain about the involvement of HS1 in the B-cell-mediated immune response.
One study has identified HS1 in the nucleus, raising the possibility that the protein mediates BCR-dependent changes in gene expression. In fact, sequence similarity between HS1 and transcription factors has led some to propose that the protein is a transcription factor.
The same study has shown that plasmacytoma cell lines do not express HS1, suggesting that terminally differentiated plasma cells express cortactin but not HS1.
The field is wide open for studies to explore these and other questions in detail.
HS1 And T-Cells
Murine HS1 was cloned and characterized as part of screening for proteins that bind to the SH3 domain of Lck kinase in T-cells; hence, its name LckBP1.
The murine gene was mapped to a region in chromosome 16 that is syntenic (functionally equivalent) to human chromosome 3q.
Both studies found that HS1 is tyrosine phosphorylated after T-cell receptor (TCR) stimulation in murine cells.
In Jurkat T-cells, however, HS1 tyrosine phosphorylation was induced in response to CD3 engagement (TCR activation) but not in response to CD28 or CD2 engagement.
A decade after the first studies of HS1 in T-cell signaling, investigators explored in depth the role of HS1 during the formation of the immunological synapse.
Those studies showed that, upon TCR ligation, HS1-deficient T-cells fail to produce a well-organized and stable F-actin assembly at the immune synapse.
HS1 is required for Ca[2+] mobilization and for IL-2 gene transcription, which is accompanied by defects in activation of NF-AT and NF-κB signaling.
Suppressing HS1 expression in T-cells still allows the exchange factor Vav1 to be recruited to the immunological synapse, but it inhibits Vav1 retention there. Therefore, HS1 has been proposed to stabilize the immunological synapse and promote Vav1 retention there.
In vitro pull-down assays performed with isolated SH2 domains indicate that HS1 binds to the SH2 domain in Lck, phospholipase C gamma 1 (PLCγ1) and Vav1.
Given that HS1 also interacts with Lck via its SH3 domain, these findings suggest that HS1 and/or phospho-HS1 can form different molecular complexes in order to achieve signal specificity.
Phospho-HS1 binds not only Lck but also c-Abl kinase through its SH2 domain, and this binding is required for full tyrosine phosphorylation of HS1 via a pathway involving the WASP member WAVE2.
The complex picture emerging from these studies suggests that many questions remain about the participation of HS1 in TCR signaling.
HS1 And Erythrocytes, Platelets, And Mast Cells
Lyn is a major SFK implicated in erythrocyte differentiation.
Two hybrid studies have shown that HS1 interacts directly via its proline-rich region with the SH3 domain of Lyn.
Expressing truncated HS1 in the J2E erythroleukaemic cell line suppressed basal and erythropoietin-induced proliferation and differentiation, clearly demonstrating the importance of HS1 for erythrocyte function.
The picture is less clear in platelets due to the fact that cortactin is also expressed in megakaryocytes and platelets.
In human mast cell cultures generated by treating umbilical cord blood cells with recombinant human stem cell factor and IL-6, cross-linking of high-affinity Fc epsilon receptor I (FcERI) induces histamine release and rapid tyrosine phosphorylation of multiple cellular substrates, including Syk and HS1.
Other studies in mast cells showed that Fer-CIP4 homology (FCH)-Bin/amphiphysin/Rvs (F-BAR) and SH2 kinase (Fes kinase) phosphorylate C-terminal tyrosine residues in HS1 that are implicated in actin stabilization.
HS1 And Natural Killer Cells
To analyze the role of non-phosphorylated HS1 and HS1 phosphorylated on tyrosines 378 and 397 in natural killer cells, one study elegantly analyzed actin accumulation at the:
- immunological synapse
- target cell lysis
- adhesion
- chemotaxis
The results showed that the phosphorylation of tyrosine 378 is required for chemotaxis.
Phosphorylation of tyrosine 397, in contrast, primarily influences adhesion processes.
It is required for:
- adhesion to the integrin ligand ICAM-1 (Intercellular Adhesion Molecule 1)
- recruitment of integrins
- adaptors to the lytic synapse
- actin polymerization
HS1 And Dendritic Cells
Dendritic cells (DCs) patrol the body in search of pathogens that they can present to other immune cells.
In addition to acting as professional antigen-presenting cells, they regulate the immune response in important ways.
They can migrate using several actin-containing structures, including podosomes.
DCs from HS1 knockout mice form podosomes with aberrant morphology and distribution on the ventral cell surface.
Intriguingly, recruitment of HS1 to podosomes requires the activity of a protein complex that includes:
Wiskott-Aldrich syndrome protein (WASP)Wiskott-Aldrich protein-interacting protein (WIP)
Since both WASP and HS1 are NPFs (nucleation-promoting factors) that stimulate actin polymerization, it is tempting to speculate that they collaborate to support actin-dependent processes in DCs.
However, WASP-deficient DCs show a migration phenotype different from HS1-deficient DCs.
Although both cell types show reduced directional persistence, WASP-deficient cells move more slowly than wild-type DCs, whereas HS1-deficient cells move faster.
This suggests that WASP and HS1 perform complementary functions in DCs.
HS1 In Immunological Diseases
Since HS1 is widely expressed in immune cells and participates in diverse signaling pathways, as outlined in the sections above, it seems likely that alterations in HS1 expression or activity should affect many immune functions.
In fact, HS1 has already been implicated in diverse pathological conditions.
Nevertheless, HS1 mutations associated with human immune deficiency have yet to be described, and this seems likely to come in the future.
Lupus
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by hyperactivation of autoreactive immune cells.
In a study of Japanese women with SLE, a mutant HS1 protein was identified with a 37-residue deletion caused by exon skipping.
The resulting protein lacked the third repeat downstream of the NTA domain. Additional polymorphisms in HS1 have been associated with SLE.
Investigators have proposed that all these mutations lead to a hyperactive protein, but this must be tested in structural and biochemical studies.
Leukemia
HS1 is an important actin regulatory protein participating in BCR signaling, raising the possibility that its malfunction is linked with cancer such as B-cell-derived leukemia.
While early studies hypothesized that cortactin expression is associated with plasma cell transformation, pioneering work from the Caligaris-Cappio laboratory in Italy has implicated HS1 in chronic lymphocytic leukemia (CLL).
Their studies indicate that in CLL patients with poor prognosis, most HS1 is constitutively phosphorylated, whereas only a fraction is phosphorylated in patients with good prognosis.
BCR stimulation increases the levels of phosphorylated HS1 in normal mature B-cells and memory B-cells above those in naïve B-cells.
More recent studies have shown that eliminating HS1 expression in B-cells impairs cytoskeletal remodeling, leading to abnormal cell adhesion and reduced cell migration.
In one set of experiments, B-cells were taken from CLL patients and HS1 expression was silenced. When the cells were injected into immune-deficient mice, they infiltrated organs to a lesser extent than did wild-type cells.
These findings suggest that HS1 helps control the lymphocyte trafficking necessary for tissue invasion by malignant B-cells in chronic lymphocytic leukemia.
In CLL involving B-cells, Lyn kinase is overexpressed and forms complexes in the cytosol with HS1.
Recent work has shown that signaling by these Lyn/HS1 complexes promotes leukemic cell survival. In addition, HS1 has been detected in the nucleus of neoplastic B cells.
These findings point to HS1 as a potential anticancer therapeutic target.
Along these lines, it is intriguing that HS1 levels in ex vivo leukemic cells from patients responsive to therapywith the purine analogue fludarabine are significantly lower than in nonresponsive patients.
HS1 has also been implicated in acute myeloid leukemia (AML).
Patients with monocytic AML frequently have mutations in the gene encoding FMS-like tyrosine kinase 3 (Flt3).
Ligands for this receptor act as growth factors for monocytic precursors.
In ligand-dependent proliferation assays of AML monocytes, both HS1 and the serine-threonine kinase Pak1/2 were found to be phosphorylated.
Neutropenia
In 1956, Dr. Rolf Kostmann (1909-1982) described a hereditary chronic neutropenia he called “infantile agranulocytosis,” which was later termed “Kostmann disease.”
Nearly 50 years passed before homozygous mutations in the gene encoding HCLS1-associated X1 (HAX-1) were found in affected individuals, including members of the original pedigree used in Kostmann’s work.
HAX-1 had previously been identified and cloned in yeast two-hybrid screening using HS1 as bait.
HAX-1 refers to a family of ubiquitously-expressed proteins, ranging in size from 26 to 35 kDa, which result from complex alternative splicing events of a single HAX-1 gene.
The protein localizes predominantly to mitochondria, and, to a lesser extent, to the:
- endoplasmic reticulum
- nuclear membrane
The primary HAX-1 function is mainly to regulate apoptosis, but it also contributes to cell migration.
HAX-1, together with HS1, is a major regulator of myeloid development and homeostasis, which may be due at least in part to the fact that HAX-1 protects myeloid cells against apoptosis.
After their seminal discovery that HAX-1 is mutated in Kostmann disease, the Welte laboratory explored the causes underlying neutropenia.
They found that granulocyte colony-stimulating factor (G-CSF) promotes HS1 phosphorylation in myeloid cells.
After stimulation by G-CSF, HS1 binds the transcription factor lymphoid-enhancer binding factor 1 (LEF-1), and the LEF-1/HS1 complex translocates into the nucleus.
This finding provides direct evidence for the nearly two-decade-old proposal that HS1 is a transcriptional factor based on the presence of “helix-turn-helix” motifs. Alterations in this signaling pathway have been described in patients with severe congenital neutropenia.
The involvement of HS1 in neutropenia may not stop here.
HS1 has been shown to localize to the leading edge of migrating neutrophils during their chemotactic response to formyl-Met-Leu-Phe (fMLP), a process involving RacGTPase.
This localization appears to require phosphorylation at tyrosines 222, 378, and 397.
Conclusion
Despite their similarities, cortactin and HS1 are different proteins with functions that do not entirely overlap.
For example, structural differences between the proteins may explain some immune-specific functions of HS1.
Future work should seek to establish whether HS1 helps bacterial and viral pathogens evade the immune system and investigate how hematopoietic lineage cell-specific protein 1 functions during normal and pathological immune responses.
Jump to
Preliminary Discussion
Domain Organization Of HS1
HS1 Tyrosine Phosphorylation
HS1 Serine/Threonine Phosphorylation
HS1 Knockout Mouse Model
HS1 And B-Cells
HS1 And T-Cells
HS1 And Erythrocytes, Platelets, And Mast Cells
HS1 And Natural Killer Cells
HS1 And Dendritic Cells
HS1 In Immunological Diseases
Conclusion

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles