Escherichia Coli - Advances, Challenges, And Recombinant Protein Expression
Recombinant proteins are created using Escherichia coli. Cell factories and expression platforms are the most widely used in the world. Several molecular tools and procedures, like as expression plasmids, modified strains, and culturing strategies, can be used to produce high-level heterologous protein productions.
Author:Suleman ShahReviewer:Han JuMay 29, 202323.8K Shares663.6K Views
Recombinant proteins are created using Escherichia coli. Cell factories and expression platforms are the most widely used in the world.
Several molecular tools and procedures, like as expression plasmids, modified strains, and culturing strategies, can be used to produce high-level heterologous protein productions.
Biochemistry has been transformed by recombinant protein synthesis in microorganisms.
Purifying little amounts of protein used to require kilos of animal and plant tissues or vast volumes of biological fluids are nearly over.
Recombinant methods are first considered by every researcher who requires a pure protein.
Biochemical characterization, industrial use, and commercialization can all be made possible by mass-producing recombinant proteins.
What Is Escherichia Coli?
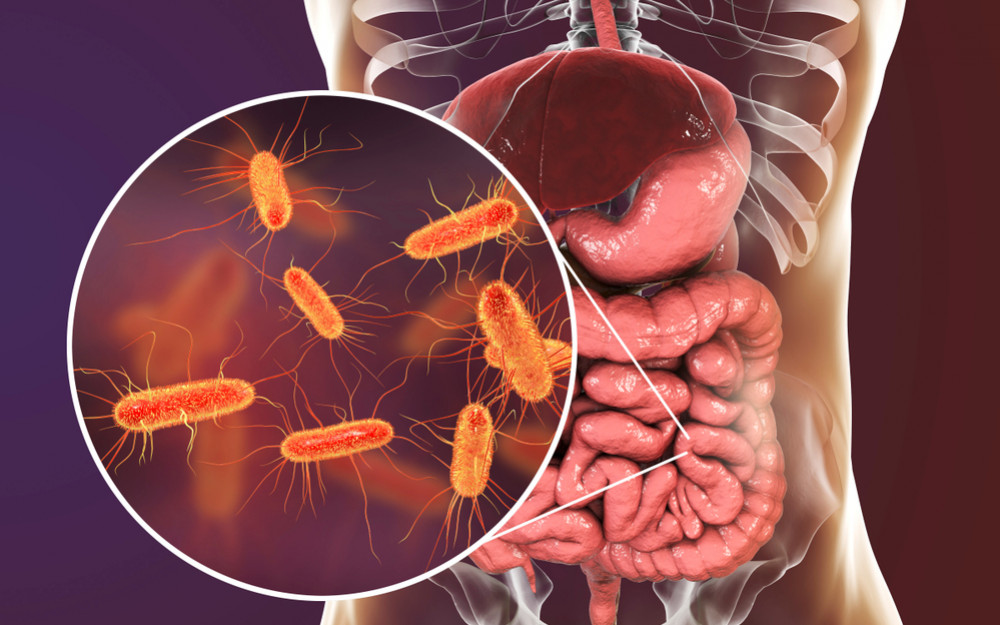
Escherichia coli (E. coli) dwells in healthy individuals and animals' intestines. Bacteria are usually harmless. Aids digestion. Certain E. coli strains can cause diarrhea, stomach pain, and fever. Dangerous E. coli infections exist. Enterobacteriaceae rod-shaped bacterium. It can survive without air. Healthy people and warm-blooded animals have these bacteria in their intestines.
What Are The Symptoms Of An Escherichia Coli Infection?
Infections with the STEC strain of E. coli can result in the following:
- Low fever.
- Vomiting.
- Loss of appetite or nausea.
- Fatigue.
- Diarrhea that may range from watery to bloody.
- Stomach pains and cramps.
E. coli Video
What Are The Different Strains Of Escherichia Coli?
Diarrhea can be caused by six different E. coli strains. These are the strains:
- Diffusely adherent E. coli (DAEC).
- Enteropathogenic E. coli (EPIC).
- Enteroinvasive E. coli (EIEC).
- Enteroaggregative E. coli (EAEC).
- Enterotoxigenic E. coli (ETEC).
- Shiga toxin-producing E. coli (STEC).
Which Organism To Use For Recombinant Protein Expression?
The process begins with the selection of the host cell whose protein synthesis machinery will create the valuable protein. It defines the project's molecular tools, equipment, and reagents. Bacteria, yeast, filamentous fungi, and unicellular algae are microorganism hosts. Their choice depends on the protein of interest. Prokaryotic expression systems may not be adequate for eukaryotic post-translational modifications (like glycosylation). E. coli is a popular host organism.
Which Plasmid Should Be Chosen?
Common expression plasmids combine replicons, promoters, selection markers, numerous cloning sites, and fusion protein/fusion protein removal techniques. It's easy to become lost in the large library of accessible expression vectors. To make an informed decision, examine these qualities based on your needs.
Replicon
Replicons are found in autonomously replicating genetic components like plasmids. It has one replication origin and cis-acting control elements. Copy number is an important vector parameter. Replicon controls copy number. High plasmid dosage equals greater recombinant protein yield since the cell has more expression units. A high plasmid number can slow bacterial growth and cause plasmid instability, reducing the amount of healthy organisms for protein synthesis. High copy number plasmids do not boost protein synthesis yields.
Promoter
The lac promoter is a standard in bacterial promoter research. The system's collected expertise allowed expression vectors. Lactose stimulates the system and is used to make protein. Induction is hard when carbon sources are easily metabolized (such as glucose present in rich media). If lactose and glucose are present, lac promoter expression isn't fully activated until all glucose is used. Low glucose produces cyclic adenosine monophosphate (cAMP), which activates the lac operon. Catabolite suppression controls expression positively. Low cAMP levels in lac operon-repressing sugars associated with reduced lac operon expression.
Selection Marker
Adding a resistance identifier to the plasmid backbone prevents plasmid-free cell development. E. coli uses antibiotic-resistant genes for this. The bla gene produces a periplasmic enzyme that inactivates ampicillin's β-lactam ring. As β-lactamase is continuously released, ampicillin is degraded and exhausted within hours. During culture, cells without the plasmid can multiply. Selective drugs with degradation-based resistance, such chloramphenicol and kanamycin, could also have this problem. Because resistant cells actively efflux the antibiotic, tetracycline is highly durable throughout cultivation.
Affinity Tags
When designing a project that requires a pure soluble active recombinant protein, it's important to detect it throughout the expression and purification scheme, (ii) achieve maximum solubility, and (iii) easily purify it from E. coli. By expressing a stretch of amino acids (peptide tag) or a big polypeptide (fusion partner) with the required protein, these three aims may be easily achieved.
Small peptide tags attached to proteins interfere less. In some situations, they may affect the fused chimeric protein's tertiary structure or biological activity. Vectors allow tagging either the N or C-terminus. If the protein's 3-D structure is available, insert the tag on the solvent-accessible end. Poly-Arg, FLAG, poly-His, c-Myc, S, and Strep II-tags are short peptide tags.

E. Coli: What You Need to Know
Tag Removal
If structural or biochemical research are needed, the fusion partner must be removed. Peptide tags can interfere with protein activity and structure, however they can be left in situ for crystallographic research. Enzymatic or chemical cleavage removes tags.
Expression vectors encode protease cleavage sites downstream of the tag gene for enzyme digestion. Enterokinase, thrombin, factor Xa, and TEV protease have been employed to remove peptide tags and fusion partners. Specificity, cost, number of amino acids left in the protein after cleavage, and digesting ease are considered while choosing proteases.
Which Is The Appropriate Host?
A fast search for a suitable E. coli strain as a host yields dozens of choices. Each has pros and cons. Many are specialist strains used for specific purposes. Only BL21(DE3) and K-12 derivatives are needed for an initial expression screen.
Daegelen et al. trace the historyof BL21 and BL21(DE3) strains. Studier described BL21 in 1986 after modifying d'Herelle's B line. BL21 has some notable genetic traits. BL21 cells lack the Lon protease, which destroys foreign proteins. Another gene absent from BL21's ancestors' genome is OmpT, which degrades extracellular proteins. Cells take up free amino acids. After cell lysis, OmpT may digest recombinant proteins. The hsdSB mutation in BL21's parent strain (B834) prevents plasmid loss. This disrupts DNA methylation and degradation. T7 RNAP should be used when the target gene is under a T7 promoter. BL21(DE3) contains the DE3 prophage, which contains the T7 RNAP gene under the lacUV5 promoter.
Which Is The Combination For Success?
It's evident that building an expression system has many alternatives. To get the required protein, many circumstances must be examined. If the project requires expressing two protein constructions in six distinct vectors, each transformed in three strains, you'll need 36 expression trials. Other variables may increase this amount. Micro-expression experiments can speed up this time-consuming trial-and-error pilot investigation. 2-ml tubes or 96-well plates are used for small-scale screening. High throughput methods using liquid handling robots allow a single person to evaluate over 1000 culture conditions in a week.
Troubleshooting Recombinant Protein Production
This article presents enhancing recombinant protein production in E. coli. Even with rigorous plasmid and host selection, it's impossible to anticipate if the protein will be in huge quantities and a soluble active form. Various situations often prevent accomplishing this goal. In each scenario, the following paragraphs offer solutions.
No Or Low Production
This is a worst-case scenario. When a protein of interest cannot be detected by a sensitive approach (e.g., Western blot) or is detected at very low levels (less than micrograms per liter of culture), it often has a detrimental effect on the cell.
Protein Toxicity
Protein toxicity occurs when recombinant protein fulfills a superfluous and harmful function in the host cell. This function interferes with the microorganism's normal proliferation and homeostasis, causing slowed growth, poor cell density, and death.
Before induction, check cell growth. If a recombinant strain grows more slowly than an empty-vector strain, gene toxicity and basal production of hazardous mRNA/protein may be to blame.
Codon Bias
Codon bias occurs when synonymous codons appear more frequently in foreign DNA than in the host. Full recombinant protein synthesis depletes low-abundance tRNAs. This defect may lead to amino acid misincorporation and/or truncation of the polypeptide, affecting heterologous protein production and/or activity. Free online programs detect uncommon codons in a gene when E. coli is employed as a host to check for codon bias while generating recombinant proteins. E. coli rarely uses rare codons (<1%). The AGG codon (Arg) is utilized seldom in E. coli but frequently in plant mRNAs (>1.5%).
Limiting Factors In Batch Cultivation
When recombinant protein expression is poor and cannot be enhanced by the proposed processes, culture density can be increased to increase volumetric protein production. Changing medium composition and vigorously shaking can produce this.
E. coli is cultured in LB. It's easy to produce, nutrient-rich, and perfect for early log phase growth. All these qualities make it suitable for protein production but not for high-density cultures. Despite the broth's richness, cell proliferation is poor. LB contains few carbs and divalent cations.
Inclusion Bodies Formation
Foreign genes in E. coli lose spatio-temporal expression control. The freshly synthesized recombinant polypeptide is expressed in E. coli, where pH, osmolarity, redox potential, cofactors, and folding processes may change from the original source. In high level expression, hydrophobic polypeptide stretches are present in high concentrations and ready for contact. This causes protein instability and aggregation. IBs are protein clumps. IBs occur when protein aggregation and solubilization are imbalanced. Soluble recombinant proteins can be obtained by reducing IB formation. One method fuses the protein to a solubility enhancer. Section "Affinity Tags" gave examples. IB can be beneficial if the protein can be refolded in vitro. If so, conditions can be modified to encourage IB production, giving a straightforward way for one-step protein purification.
Disulfide Bond Formation
Correct disulfide bond formation is crucial for many recombinant proteins' physiologically active three-dimensional structure. Erroneous disulfide links can cause protein misfolding and IB. In E. coli, cysteine oxidation occurs in the periplasm, where enzymes from the Dsb family catalyze disulfide exchange processes. Disulfide bond production in the cytoplasm is rare, possibly because cysteine residues are enzyme catalytic sites. Protein inactivation, misfolding, and aggregation can result from disulfide bond formation. The thioredoxin–thioredoxin reductase (trxB) and glutaredoxin–glutaredoxin reductase (gor) systems maintain the cytoplasm's negative redox potential and reducing environment. This affects the synthesis of disulfide-bonded recombinant proteins. As stated in "Protein Toxicity," one method is to target the periplasm.
Chaperone Co-Expression And Cofactor Supplementation
Molecular chaperones help nascent polypeptides reach their ultimate structure. ClpB can deconstruct unfolded IB polypeptides. High levels of recombinant protein expression overload the cytosol, which can overwhelm quality control mechanisms. To cease protein expression, remove the inducer after centrifugation and apply chloramphenicol-supplemented new medium. This permits molecular chaperones to help fold recombinant polypeptides.
Slowing Down Production Rate
Slower protein production lets recombinant proteins fold appropriately. This was covered when we examined translational pauses at uncommon codons and recombinant protein production. Reducing protein concentration improves folding. Increasing incubation temperature is the most common strategy to slow protein production. Temperature-dependent hydrophobic interactions enhance aggregation at higher temperatures.
Protein Inactivity
Having soluble protein isn't enough, the protein may still be poor, meaning it lacks action. Unfinished folding may be to blame and the protein adopts a stable soluble shape, however the active site design is inadequate. Some already-mentioned options can help, some proteins fold using tiny molecules or prosthetic groups.
Adding these chemicals to culture medium increases protein yield and quality. Incorrect disulfide bond formation can inactivate proteins. Low-temperature protein manufacturing affects quality.
According to discoveries by a lab, it was found that reducing the culture temperature improves the structural quality and functionality of recombinant proteins. This happened when intracellular DnaK content was high, and this phenomenon brings solubility's quality into doubt. Based on this, it may be advisable to express all recombinant proteins at low temperatures or to compare their specific activities.
People Also Ask
How Do You Treat Escherichia Coli?
A cure, relief of symptoms or prevention of consequences are not possible with existing therapy. Relaxation and sleep are the most common forms of treatment for the vast majority of people. To avoid dehydration and exhaustion, drink plenty of water.
How Did I Get Escherichia Coli In My Urinary Tract?
Stool is a common entry point for E. coli bacteria entering the urinary tract. Due to the proximity of the urethra to the anus, women are more susceptible to UTIs than men. A woman's urinary tract is also shorter than a man's, allowing bacteria better access to the bladder, which accounts for the vast majority of UTIs.
Can Escherichia Coli Spread From Person To Person?
Normal, every-day contacts with friends and neighbors do not spread E. Coli, such as coughing or kissing. As a result, hand-to-mouth contact can spread the infection from person to person after someone has consumed contaminated food or water.
How Long Does It Take For Escherichia Coli To Go Away?
Within five to ten days, most people recover without therapy from E. coli infection. This illness should not be treated with antibiotics due to the possibility of kidney problems. It's also a good idea to avoid antidiarrheal medications.
Concluding
Escherichia coli is the ideal cell factory for recombinant expression. E. coli can express prokaryotic and eukaryotic globular proteins. Several papers have achieved success expressing membrane proteins and proteins above 60 kDa. Less than 50% of bacterial proteins and less than 15% of non-bacterial proteins can be expressed in E. coli in a soluble form, demonstrating the system's flexibility. Things can get problematic when expressing a challenging protein. We hope to have provided a comprehensive list of E. coli protein expression solutions, but be careful. Many of the tactics outlined here will fail. This is because tactics for debugging recombinant protein expression are sometimes protein-specific and suffer from positive bias; i.e., only successful strategies are publicized.
Jump to
What Is Escherichia Coli?
What Are The Symptoms Of An Escherichia Coli Infection?
What Are The Different Strains Of Escherichia Coli?
Which Organism To Use For Recombinant Protein Expression?
Which Plasmid Should Be Chosen?
Which Is The Appropriate Host?
Which Is The Combination For Success?
Troubleshooting Recombinant Protein Production
Protein Inactivity
People Also Ask
Concluding

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles