Lipid Rafts - What Are They And Why Do They Matter? [Essential Insights]
In the initiation of many pharmacological agent-induced signaling pathways, lipid rafts play an important role. They’re microdomains of the plasma membrane enriched in cholesterol.
Author:Suleman ShahReviewer:Han JuAug 20, 202418.9K Shares422.1K Views

Basophils or mast cells play roles in the pathophysiology of allergic asthma, and cluster formation of lipid raftswith cross-linking of high-affinity immunoglobulin E (IgE) receptor contributes to the activation of basophils or mast cells and the process of granule exocytosis.
Anti-asthmatic drugs, such as glucocorticoids and beta-2 (β2) agonists, inhibit cluster formation of lipid rafts, via mobility of the membrane and internalization of beta-2-adrenergic receptors, respectively.
The term “lipid raft” was first introduced by Kai Simons and Elina Ikonen in their study published in 1997 by the journal Natureto describe specialized liquid-ordered membrane microdomains that are enriched in cholesterol and sphingolipids.
Lipid rafts have been defined as “small (10 to 200 nanometers) heterogeneous membrane domains, termed nanoclusters, that are involved in the compartmentalization of various cellular processes.”
Many surface receptors are constitutively or inducibly associated with lipid rafts, and it has been suggested that many multicomponent signaling pathways are coordinated by colocalization in lipid rafts, including these signaling pathways:
- immunoglobulin E
- T-cell antigen receptor
- G protein-coupled receptor (GPCR)
Downstream these signaling pathways lead to the induction of cytokines in lymphocytes and granule secretion, including histamine.
Asthma management is focused on achieving and maintaining asthma control.
The international asthma management guidelines recommend an inhaled corticosteroid (ICS) as the first step for maintaining asthma control and a long-acting β2-agonist (LABA) as add-on therapyin patients with asthma.
In this review, we will present evidence to support that the efficacy of asthma drugs, ICS, and LABA occurs through raft-dependent exocytosis and endocytosis in basophils or mast cells, which are specialized secretory cells.
Lipid Raft And Exocytosis
Basophils or mast cells play an important role in allergic and autoimmune diseases.
Lipid rafts contribute to the early phase of regulation of basophil or mast cell activation through cross-linking of IgE and triggering of granule exocytosis.
Cross-linking of the plasma membrane high-affinity IgE receptors (FcεRI) by antigens is required for basophil or mast cell activation and involves recruitment of receptor-associated tyrosine kinases to lipid rafts.
Cross-linking of IgE induces Lyn phosphorylation.
Flotillin-1, which is localized in lipid rafts, is involved in the process of phosphorylation of Lyn.
Upon activation through the FcεRI, basophils or mast cells can release up to 100% of their content of preformed mediators from cytoplasmic secretory granules by compound exocytosis, through fusion with the plasma membrane.
When purified human basophils were isolated from the blood of house dust mite, antigen-sensitive donors and stimulated with house dust mite, anti-IgE aggregated on the cells, and histamine was secreted via exocytosis.
However, glucocorticoids did not inhibit cluster formation of IgE crosslinking on house dust mite-treated basophils.
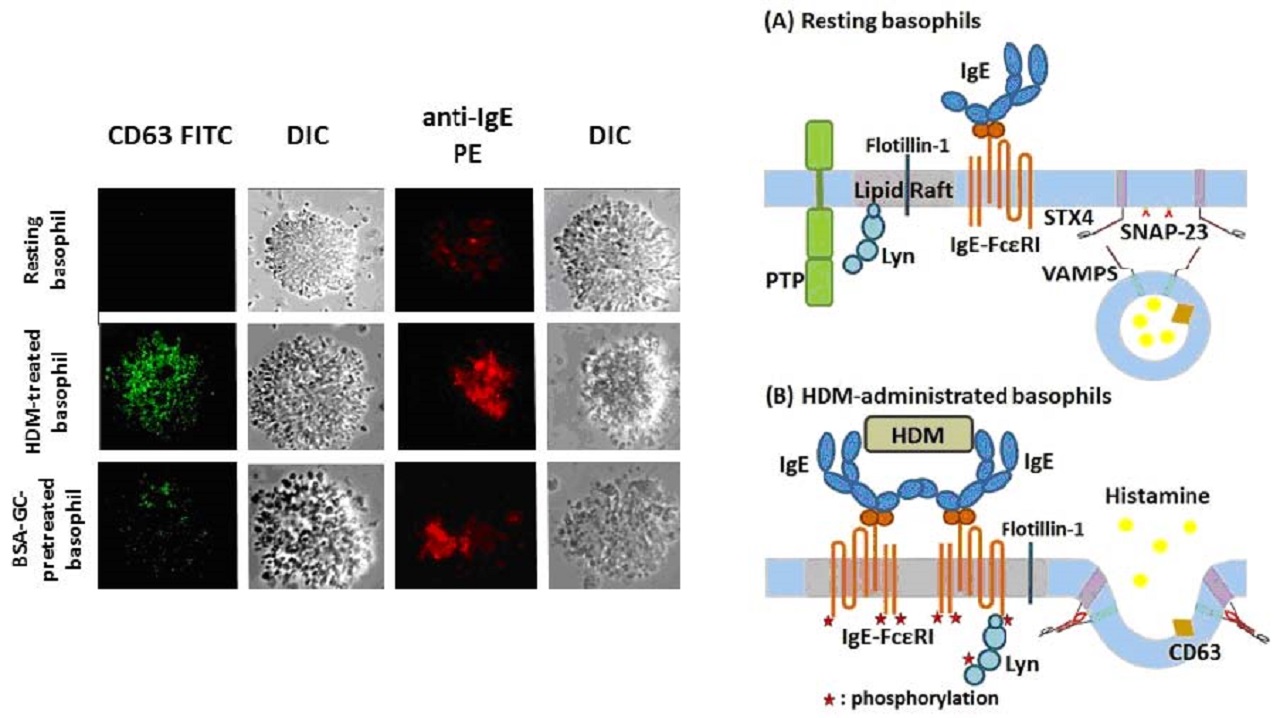
After cross-linking of FcεRI, the process of granule exocytosis, including translocation of the vesicle, docking and fusion to the inner membrane, occurs successively on grounded lipid rafts.
The soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) family of membrane fusion proteins, including synaptosomal-associated protein 23 (SNAP-23) and vesicle-associated membrane proteins (VAMPS), plays a role in granule exocytosis.
Recent work has uncovered a central role of SNARE proteins, which are present on both granules and the plasma membrane, in regulating the fusion of these granules with the plasma membrane of basophils or mast cells during exocytosis.
Since SNARE proteins are partly localized in lipid rafts, inhibition of cluster formation of lipid rafts by glucocorticoids might prevent granule exocytosis.
Lipid Raft And Non-Genomic Inhibitory Effects Of GCs
Glucocorticoids (GCs) regulate various kinds of inflammatory cells through their anti-inflammatory effects via both genomic and non-genomic means.
Among the non-genomic effects, glucocorticoids exhibit rapid onset and therefore occur through acute regulation of intracellular signaling cascades.
Several studies have reported that non-genomic effects contribute to glucocorticoid inhibition of granule exocytosis by basophils or mast cells.
Because membrane-impermeable bovine serum albumin (BSA)-conjugated glucocorticoid exhibits the same effect as non-conjugated glucocorticoid, the inhibitory effects have been suggested to occur, at least in part, through binding to membrane-bound glucocorticoid receptors (mGRs).
Non-genomic inhibitory effects of glucocorticoids through suppression of cluster formation of lipid rafts lead to inhibition of granule exocytosis from basophils or mast cells.
Pretreatment with glucocorticoids inhibits the expression of mGR and GM1 gangliosides, which are enriched in lipid rafts and can be detected using a probe of cholera toxin-B (CT-b), in house dust mite-treated basophils from house dust mite-sensitive subjects.
Non-genomic mechanisms are involved in the rapid inhibitory effect of glucocorticoids on cluster formation of lipid rafts, through binding to mGRs on the plasma membranes of activated basophils.
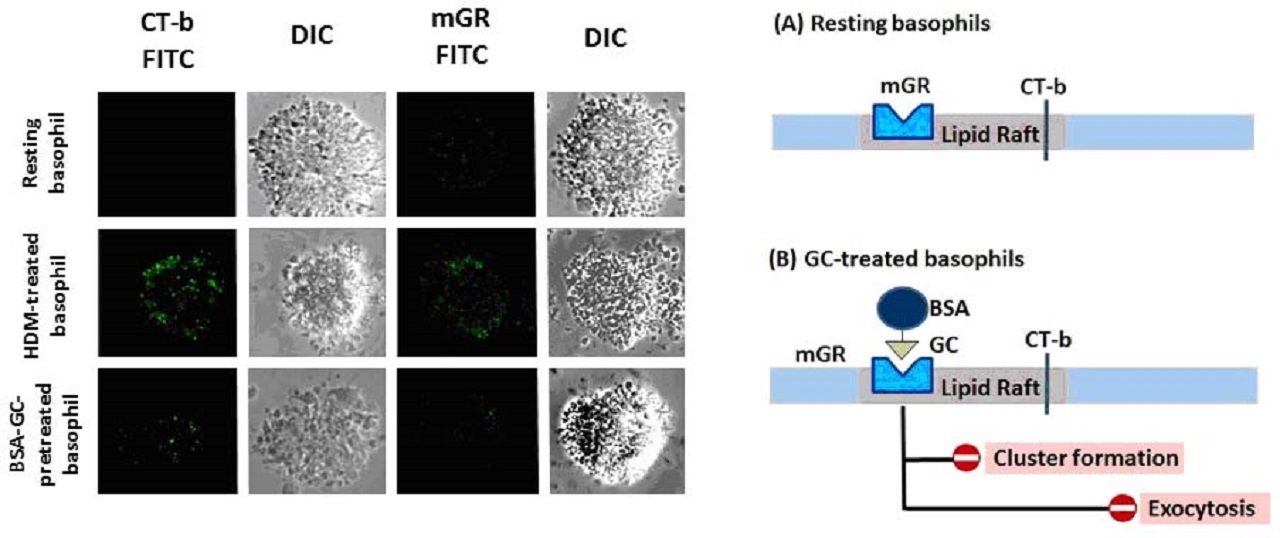
Caveolae are caveolin-1-enriched, smooth invaginations of the plasma membrane that form a subdomain of lipid rafts. Caveolae have been detected in the microvilli and intracellular vesicles in mast cells.
Coimmunoprecipitation studies have identified interactions between mGRs and caveolin and have suggested that the activation function 1 domain within the mGR may support an interaction between mGR and caveolin.
The mechanisms by which glucocorticoids inhibit granule exocytosis are not well understood.
Cholesterol serves as a spacer between the saturated chains of sphingolipids and is essential for maintaining the liquid-ordered phase of rafts and sequestering the embedded proteins from the rest of the membrane.
Cholesterol and phospholipids play an important role in maintaining the proper fluidity and rigidity of plasma membranes.
Cholesterol-rich microdomains exhibit slower mobility in the plasma membrane than non-raft regions.
As small changes in the plasma membrane cholesterol content near the physiological set point may alter a variety of large biological responses, glucocorticoids might regulate the fluidity and rigidity of the plasma membrane.
Lipid Raft And Endocytosis Of Β2-Adrenergic Receptors
The principal action of β2-agonists is relaxation of airway smooth muscle through stimulation of β2-adrenergic receptors (β2ARs).
This increases the intracellular messenger cyclic adenosine monophosphate (AMP) that is responsible for the control of smooth muscle tone.
In contrast, secretory events in cells are generally accompanied by decreased levels of cyclic AMP. In mast cells, histamine release is associated with a fall in cAMP.
Fenoterol, a LABA (long-acting beta2-agonist) with a 12-hour duration of action that was recently introduced to treat asthma, inhibits antigen-induced histamine release from basophils or mast cells in a dose-dependent fashion with concomitant increases in cAMP levels.
Fenoterol also suppresses cluster formation of lipid rafts, thus inhibiting granule exocytosis from basophils or mast cells.
β2-agonist activation of β2AR, which is a prototypical member of the GPCR (G protein-coupled receptor) family, leads to conformational changes that result in coupling to G protein (guanine nucleotide-binding protein), aka GTPase (guanosine triphosphate), which, in turn, generates cAMP as a second messenger.
The activated β2AR is then phosphorylated, resulting in the binding of β-arrestin that physically interdicts further G protein coupling leading to receptor desensitization.
The phosphorylated β2AR is internalized and undergoes resensitization by dephosphorylation mediated by protein phosphatase 2A in the early endosomes.
Among the mechanisms regulating heterotrimeric G protein internalization, the majority of GPCRs are trafficked into clathrin-coated pits and internalized by endocytosis.
Upon agonist binding, β2ARs are rapidly internalized by endocytosis into clathrin-coated pits, and they traffic into recycling endosomes.
Lipid rafts and caveolae are also specialized membrane microdomains that have been implicated in regulating GPCR signaling cascades and the regulation of β2AR/Gα(s) signaling.
Because Gαs is internalized with the raft-dependent endocytic pathway of cholera toxin-B (CT-b), which has also been characterized as a dynamin-dependent, caveolar pathway, agonist-induced internalization of Gαs appears to be dynamin 1-dependent through non-clathrin–mediated endocytosis.
Pretreatment with β2-agonists inhibits the expression of fluorescent CT-b in house dust mite-treated basophils obtained from house dust mite-sensitive subjects.
The inhibition of cluster formation of CT-b implies internalization of Gαs through the calveolar-dependent, clathrin-coated pit-independent pathway; consequently, inhibiting of tracking Gαs into the cytoplasm might inhibit G protein/GTPase tubulin and microtubule dynamics leading to suppression of granule exocytosis.
Future Of Drug And Lipid Rafts
Cluster formation of lipid rafts with crosslinking of FcεRI contributes to the activation of basophils or mast cells during the process of exocytosis.
Anti-asthmatic drugs, glucocorticoids and β2-agonists, do not inhibit cluster formation of IgE cross-linking.
As the structure of glucocorticoids might stabilize the fluidity of the plasma membrane, a new glucocorticoid drug could exploit the ability to stabilize the plasma membrane and inhibit granule exocytosis.
Furthermore, internalization of β2AR is also associated with clinical desensitization, which implies that chronic treatment with β2-agonists may lead to deterioration in lung function.
A new β2-agonist drug is needed in view of inhibiting desensitization of Gαs.
A variety of beta2-agonists with long half-lives, also called ultra long-acting beta2-agonists (LABA), are currently under development with the hope of achieving once-daily dosing.
It has been hypothesized that the long duration of action of indacaterol may be related to its high affinity for the lipid raft domain of the plasma membrane.
Acetylcholine is involved in the control of airway smooth muscle constriction and in recruitment of inflammatory cells, via neuronal and paracrine effects on muscarinic type 3 (M3) receptors.
Long-acting muscarinic antagonists (LAMAs) are well established in the guidelines for the treatment of chronic obstructive pulmonary disease (COPD) but are not currently licensed for use in asthma.
M3 muscarinic receptors, as well as β2ARs, enter cells constitutively by clathrin-independent endocytosis and colocalize with markers of this endosomal pathway on recycling tubular endosomes.
Muscarinic agonists promote histamine release via the M1-mediated pathway on basophils or mast cells.
Conclusion
Future work will be required to determine whether long-acting muscarinic antagonists (LAMAs) could inhibit cluster formation of lipid rafts on basophils or mast cells, as well as smooth muscle and gland cells.
In addition, further studies should be conducted about lipid rafts in relation to anti-asthma medications.

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles