Does Lymphatic Growth Rely On Immune Cell Function?
In this review, we have reported the newly described impacts of immune cells on lymphatic growth and function in developmental and pathological contexts related to inflammatory events.
Author:Suleman ShahReviewer:Han JuSep 24, 202420.8K Shares434.6K Views
Lymphatic vessels found in most of the tissues in the body form a network that is tremendously important in macromolecular and cellular transport from peripheral tissues.
In the past decade, a massive number of studies have focused on the role of the lymphatic system in immune cell trafficking during both physiological and pathological conditions.
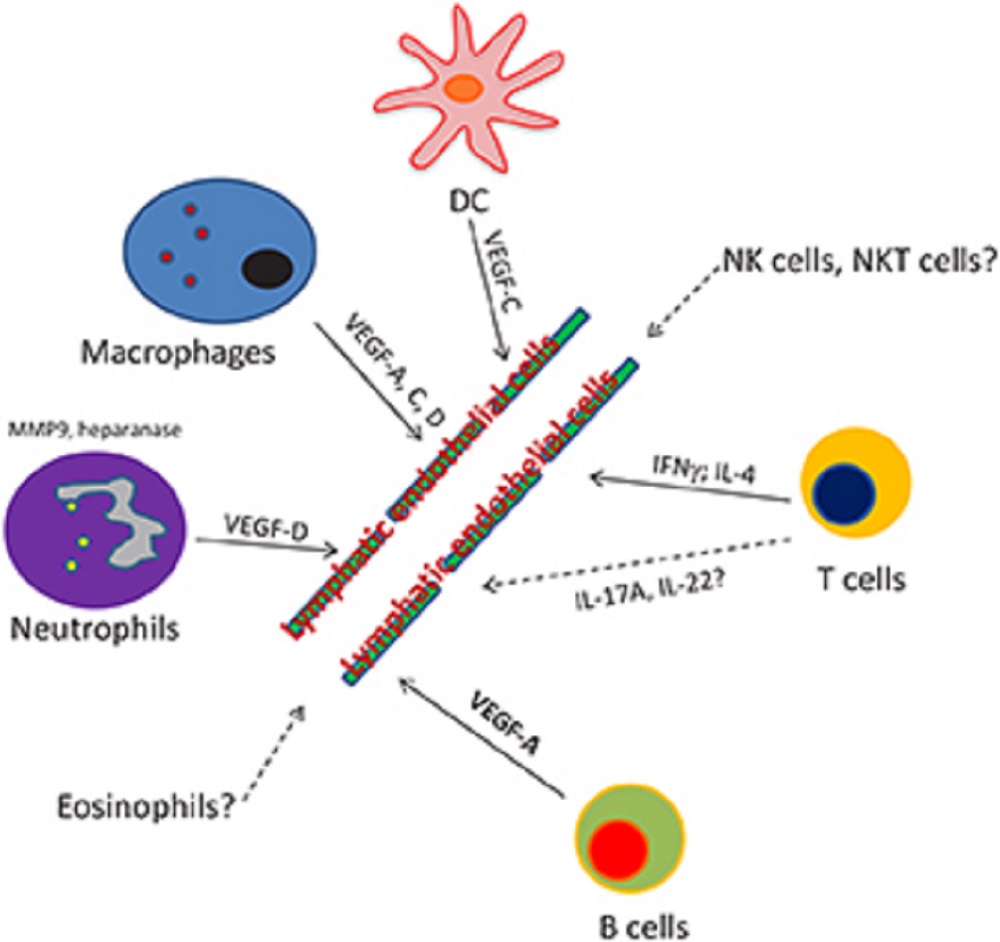
Concomitantly, immune cells, such as dendritic cells, monocytes, and T and B lymphocytes, have been described to be the major source of lymphangiogenic factors.
Put together, it has been hypothesized that immune cells not only travel through lymphatic vessels from point A to point B but also regulate lymphatic vessel expansion and function per se.
Several findings suggest a convincing give-and-take interaction between the circulating immune cells and the lymphatic vessels.
Lymphatic Network
The lymphatic network is part of a system that extends its vessels in nearly every tissue of the body.
The main functions of this tightly regulated system are to allow fluid hemostasis and to permit immune cell trafficking and lipid transport.
In addition, the physiological relevance of the lymphatic circulation in removing cholesterol from peripheral tissues has been recently described.
Whether lymphatic network expansion is a friend or foe in the onset and/or progression of numerous diseases still requires attention.
In the past decade, a growing number of studies have been exploring the “regulation of lymphangiogenesis” not only during developmental, but also inflammatory settings, together with the pleiotropic activities of several classes of immune cells on the regulation of lymphangiogenesis during pathological and non-pathological contexts.
In this review, we focus on the roles of immune cells, such as macrophages, B and T lymphocytes, and dendritic cells (DCs), played in lymphangiogenesis and lymphatic vessels remodeling, during developmental and pathological contexts related to inflammatory events.
Lymphangiogenic Factors
Lymphangiogenesis during development and inflammationis a tightly regulated process depending on several specific transcription factors, including the vascular endothelial growth factors (VEGF) in:
- Prox1 (prospero-related homeobox protein 1)
- SOX18 (SRY-related HMG-box or SOX)
- chicken ovalbumin upstream promoter transcription factor II or COUP-TFII(1-3)
VEGF-A, C, and D play a critical role in the development of the lymphatic system through the binding and activation of VEGFR-2 and 3 signaling.
VEGF-A exercises its biological function through binding and signaling via VEGFR-2, whereas VEGF-C and D act via VEGFR-3.
As immune cells are the main source of lymphangiogenic factors, it has been predicted that modulating these cells’ function could:
- have a strong impact on lymphangiogenesis
- result in a defective lymphatic network
T Lymphocytes
Although other immune cell types are known to positively correlate with lymphangiogenesis, recent reports demonstrate that T cells are negatively regulating the development of lymphatic vessels.
In athymic nude mice, in which functional T cells are absent, a high density of Lyve1[+] lymphatic vessels can be observed in the lymph nodes.
In a study published in 2011 by the journal Immunity, the authors, with Raghu P. Kataru as lead author, show in this model that adoptive transfer of naive CD4 + or CD8 + T cells abrogates the lymph node lymphatic vessel density, a consequence that was even more prominent when activated T cells were injected.
But how can T cells, in both inflammatory and steady state, mediate this negative regulation?
Because adoptively transferred interferon-γ (IFNγ)-deficient Tcells failed to negatively regulate lymphatic vessel formation, Kataru et al. state that IFNγ is responsible for the crucial role of T cells in maintaining the homeostatic balance of lymphatic vessels, at least in the lymph nodes.
To better characterize the inflammatory response occurring in secondary lymphoedema, the authors of a study published in 2012 by the American Journal of Physiology-Cell Physiology, with Jamie C. Zampell as lead author, used a model of mouse-tail lymphoedema and antibody depletion techniques.
They assessed a diminished propensity to develop tail edema and lymphatic stasis in absence of CD4+ T cells, but not CD8+ T cells.
In CD4-depleted mice, the lymphatic function was improved, while production of IFNγ (interferon-gamma) TGF β1 (transforming growth factor beta 1 or TGFB1) was decreased, thereby increasing inflammatory lymphangiogenesis.
Those mice failed to develop lymphoedema and maintained normal lymphatic function.
In a subsequent publication, the authors of a study published in 2013 by the Federation of American Societies for Experimental Biology (FASEB), with Tomer Avraham as lead author, demonstrate that progression of lymphoedema is associated with high production of Th2-related cytokines (IL-4 and IL-13).
According to their findings, blocking those cytokine functions by using neutralizing antibodies would improve lymphatic function and inhibit the consequent inflammation and fibrosis.
Furthermore, when immunizing the mice by complete Freund’s adjuvant (CFA)/ovalbumin intradermal injection, IL-4 neutralization significantly increased lymphatic vessel density in the corresponding draining lymph node.
All together, these data demonstrate that both CD4 + and CD8 + T cells negatively regulate lymphatic vessel growth via production of soluble factors, such as IFNγ and IL-4.
Dendritic Cells
Lymphatic entry of dendritic cells has been studied extensively for more than a decade.
As dendritic cells have a crucial role in the initiation of immune responses within the draining lymph nodes, the afferent lymphatic vessels serve as active conduits by which dendritic cell migration from peripheral tissues is tightly regulated.
More recently, in their two separate studies both published in 2013 by the journal Angiogenesisand by the Journal of Cell Science, Louise A. Johnson and David G. Jackson have shown evidence that numerous chemokines and adhesion molecules are involved in a multistep process regulating the entry of dendritic cells to activated lymphatic vessels.
Since the dendritic cells must cross the endothelial barrier to enter the lymphatics, interactions occur between dendritic cells and the lymphatic endothelium.
Thus, in addition to the role of the lymphatic vessels in the transport of those key players in immunity, is it possible that there could be “give-and-take” interactions between dendritic cells and the lymphatic vessels?
In their study published in 2013 by the journal Lymphatic Research and Biology, Erica Russo, Maximilian Nitschke, and Cornelia Halin describe the current state of knowledge about the cellular interactions that occur between dendritic cells and lymphatic endothelial cells (LECs).
They summarize our current knowledge on the impact of dendritic cells/LECs interactions on the process of dendritic cell migration through the lymphatics and, of great interest, on lymphatic vessels growth.
B Lymphocytes
Is there a role for B cells in lymphangiogenesis occurring during inflammation?
In a study published in 2006 by the journal Immunity, the authors, with Veronique Angeli as lead author, have unraveled the interrelationship among:
- B cells
- lymphatic vessels
- migratory dendritic cells
Their interrelationship demonstrates that dendritic cell migration from the periphery is amplified by B cell-dependent signals.
In a mouse model of immunization with keyhole limpet hemocyanin/complete Freund’s adjuvant (CFA), they have shown that B cells are key actors in lymph node swelling.
While assessing the consequences of a lack of B cells on the regulation of factors known to promote lymphangiogenesis, Angeli et al. described that the production of VEGF-A was dramatically decreased in both immunized mice and μMT mice, lacking B cells.
In agreement with this early report, VEGF-A transgenic mice, overexpressing VEGF-A have increased number of lymphatic endothelial cell in lymph nodes and specific overexpression of VEGF-A by B cells (CD19Cre/hVEGF-Afl mice) induced increased lymphangiogenesis and angiogenesis in lymph nodes.
Despite these interesting findings on the connection between B cells and VEGF-A in lymphangiogenesis during immunization, the role of B cells and B cell-derived factors on the development of lymphatic vessels in steady state is still unclear: no direct characterization of the lymphangiogenic pattern has been assessed in mice lacking B cells compared with wild-type mice.
Comparing the regulation of lymphatic growth in wild-type mice and in mice lacking B cells (μMT or injected with a neutralizing antibody directed against CD20, for instance) would provide further insights on the role of B cells in lymphangiogenesis.
Macrophages
Macrophages constitute an important cell population typically involved in the host defense against pathogens and wound repair.
As postnatal lymphangiogenesis is primarily associated with chronic inflammation, a process that is predominantly regulated, at the cellular level, by macrophages, it is not surprising that a growing body of evidence is revealing new functions of macrophages in lymphangiogenesis.
In a study published in 2007 by the journal Arthritis Research & Therapy, the authors, with Tetsuo Kubota as lead author, described a reduction of the lymphatic network in trachea and ears in osteopetrotic mice bearing a mutation in CSF1 (colony stimulating factor 1) gene and therefore, a decreased abundance of tissue resident macrophages.
The recruitment of macrophages (VEGF-A dependent) to the site of injury, and their production of VEGF-C and VEGF-D, plays a critical step in lymphangiogenesis during inflammation.
Depletion of macrophages with clodronate liposomes strongly inhibits the formation of new lymphatic vessels.
Neutrophils
The role of neutrophils during lymphangiogenesis was not addressed until very recently.
A recent report elegantly demonstrated the role of these cells during inflammatory lymphangiogenesis.
In a study published in 2013 by the journal Blood, the authors, with Kar Wai Tan as lead author, reveal that, in B cells-deficient animals, neutrophils take over the role of B cells and orchestrate the development of the lymphatic network.
They further emphasize the function for neutrophils as organizers of lymphangiogenesis during inflammation by depleting neutrophils in wild-type mice developing skin inflammation in response to immunization or contact hypersensitization.
In this model, they show that lymphangiogenesis is decreased and local inflammation is increased.
According to their findings, neutrophil-derived matrix metalloproteinase 9 (MMP-9) and heparanase are the main factors involved in this phenomenon, by controlling VEGF-A bioavailability in the inflamed tissues.
Moreover, as neutrophils can secrete lymphangiogenic factors, notably VEGF-D, they consequently directly regulate lymphangiogenesis.
Perspectives
Despite the tremendous recent progress that we described herein, the role of immune cells during lymphangiogenesis remains poorly understood: very little is still known on the direct and indirect impact of immune cells on the development of the lymphatic network.
For instance, we reported studies showing that soluble factors such as IFNγ (interferon-gamma) and IL-4 (interleukin 4) play a critical role during both developmental and inflammatory lymphangiogenesis.
As IFNγ cannot only be produced from conventional T cells, as described, but also by innate lymphocyte populations such as natural killer (NK) cells and NK T cells, it would be interesting to point out the effect of NK and NK T cells on lymphatic growth.
Mouse models and depleting antibodies are currently available to specifically address the role of these cells during lymphangiogenesis.
Other cell types are also understudied in the process: the role of Th17 cytokines or γδ T cells, major cell population involved in the production of IL-17 and IL-22, and on lymphatic endothelial cells is also unknown.
Considering the role of eosinophils, a population of cells found in blood and tissues in both human and mouse, in modulating macrophage functions, we hypothesize that this cell type could also modulate the production of pro-lymphangiogenic signals.
Conclusion
We believe that mutual interactions between the lymphatic vessels and immune cells is critical.
In addition, understanding the specific role of different populations of immune cells during lymphangiogenesis may provide a useful approach for modulating the lymphatic network in inflammatory diseases.
More studies should be conducted regarding lymphatic growth and immune cells.

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles