How To Interpret PaO2/FIO2 In Acute Respiratory Distress Syndrome [Best Practices]
The pressure of arterial oxygen to fractional inspired oxygen concentration ratio is a commonly used indicator of lung function in critically ill patients, particularly those diagnosed with ARDS.
Author:Suleman ShahReviewer:Han JuNov 18, 2024277 Shares92.2K Views
Let us make sense of pressure of arterial oxygen to fractional inspired oxygen concentration(PaO2/FIO2).
For many years, physicians have relied on it to define and characterize the severity of the acute respiratory distress syndrome (ARDS), and this ratio is still a central element of the new ARDS definition (Berlin definition).
In addition, clinicians utilize this ratio to:
- track change in lung conditions
- set positive end expiratory pressure
- assess the response to different ventilatory strategies; and/or
- make decisions regarding the requirement for advanced supportive treatment modalities
Examples of such supportive modalities include:
- paralysis
- prone position
- extracorporeal membrane oxygenation (ECMO)
Despite having the merit of simplicity and availability, the PaO2/FIO2 is more complex to interpret than being acknowledged and can at times be misleading.
This risk is particularly present if one does not understand or consider the key determinants of the PaO2/FIO2 ratio in each individual patient and why this ratio may change over time.
Here we review the main determinants of PaO2/FIO2 ratio and discuss how the application of a few physiological key concepts can be used to optimize the management of patients with hypoxic respiratory failure.
Preliminary Discussion
In the absence of a direct reliable marker of lung injury, gas exchange is commonly used to define respiratory failure (e.g., hypoxic versus hypercapnic), as well as the degree of lung dysfunction/injury (e.g., mild to severe acute respiratory distress syndrome or ARDS).
For hypoxic respiratory failure, in general, and ARDS, in particular, the pressure of arterial oxygen to fractional inspired oxygen concentration (PaO2/FIO2) ratio is the most commonly reported index of gas exchange impairment and is a central element of the ARDS definition.
The recent revised definition (the so-called Berlin definition) has not changed its importance as the PaO2/FIO2 ratio is still required to define ARDS and to characterize its severity (mild, moderate, or severe).
This ratio is also used to identify the ARDS population, who is the most likely to benefit from specific supportive modalities, such as prone positioning or paralysis.
Finally, this ratio is also commonly calculated at the bedside to track the course of ARDS or the response to specific intervention, and to help set positive end expiratory pressure (PEEP) levels in individual patients.
Despite its simplicity and availability, the PaO2/FIO2 ratio can be complex to interpret and misleading at the bedside if one does not understand or consider its various physiological determinants.
PaO2/FIO2 And ARDS Definition
We will define pressure of arterial oxygen to fractional inspired oxygen concentration (PaO2/FIO2) and the acute respiratory distress syndrome definition (ARDS).
A detailed discussion of the Berlin definition of ARDS is beyond the scope of this critical review. However, it is relevant to stress the fact that the new ARDS definition mandates this ratio to be less than 300.
In addition, the assessment of ARDS severity relies entirely on this ratio:
- ≤300 and >200 - mild ARDS
- ≤200 and >100 - moderate ARDS
- ≤100 - severe ARDS
It is measured on a PEEP ≥5 cm. H2O.
Although the mild, moderate, and severe ARDS of the Berlin definition were found to be associated with different mortality (27%; 95% confidence interval [CI], 24% to 30%; 32%; 95% CI, 29% to 34%; and 45%; 95% CI, 42% to 48%, respectively; p < 0.001) and with increased median duration of mechanical ventilation in survivors, it is important to emphasize that such association cannot be extrapolated to individual patients.
In addition, the definition lacks standardization in regard to the key determinants of PaO2/FIO2, such as the FIO2 and PEEP used to measure PaO2. This is problematic for reasons discussed below.
Determinant Of PaO2/FIO2 Ratio In Patients With ARDS
To appreciate the limitation of applying this ratio to characterize the degree of lung injury in a given patient and to understand that sometimes this ratio changes over time irrespective of the degree of lung injury, it is important to review the key clinical factors that determine the PaO2/FIO2 ratio in the critically ill patients.
In a healthy individual, the main determinant of the PaO2 is the alveolar O2 content (PAO2).
When the ventilation to perfusion (V/Q) ratio is close to 1 and therefore only a negligible shunt (perfusion of non-ventilated lung units) is present, the venous blood becomes fully oxygenated and largely independent of the mixed venous O2 content as the PaO2 becomes identical to the PAO2.
One of the hallmarks of ARDS is the presence of shunt.
Shunt and venous admixtures are used here interchangeably to express the calculated fraction of the cardiac output that bypasses the alveolar units and that contributes to the mixing of the poorly oxygenated venous blood with the capillary oxygenated one.
In contrast to the PaO2 of the healthy individuals, the one of ARDS patients varies significantly with the degree of V/Q mismatch and/or shunt present and the mixed venous blood O2 content.
As the shunt increases, the PaO2 tends to become less and less sensitive to the PAO2 and to the FIO2 and more and more dependent on the mixed venous O2 content and saturation.
This is due to the fact that the arterial and venous O2 blood content and saturation tend to become more and more alike as the shunt fraction increases.
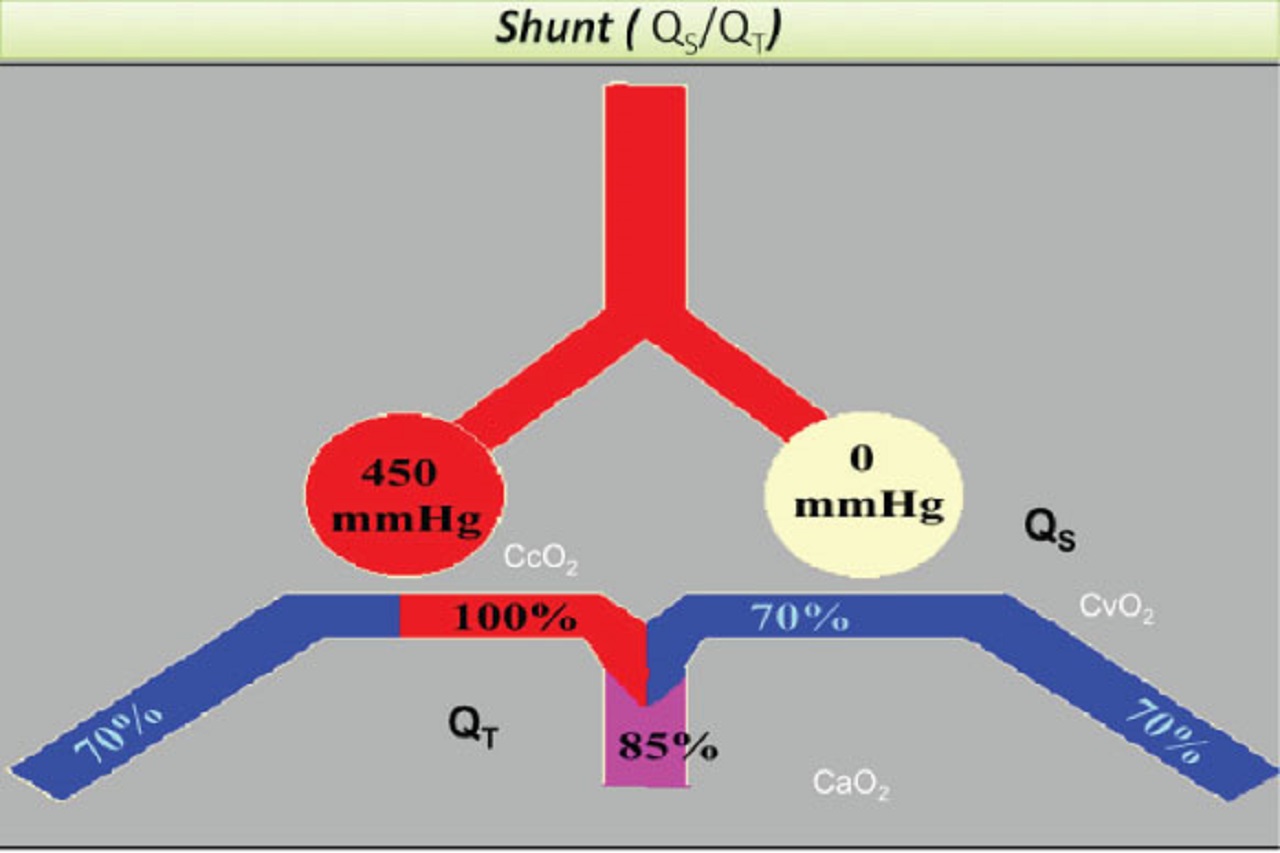
The degree of shunt can be viewed as determined and modulated by factors that affect the number of alveolar units that are non-ventilated (V factors) and/or the perfusion of those units (Q factors), as we shall now discuss.
Ventilation (V) Factors
In patients with ARDS, non-aerated alveolar units contributing to shunt physiology are mainly the result of alveolar flooding (by edema, pus, or blood) or collapse.
Although some units can be easily recruited (e.g., collapsed alveoli) by increasing the transpulmonary pressure, othersresist recruitment (e.g., alveoli are filled with pus in the setting of a pneumonia).
As a result, the degree of recruitable lung units varies among patients with ARDS and within the same patient over the course of the disease (more recruitable lung early and less later on).
This has important implications.
Firstly, depending on the ventilatory strategy used and the degree of recruitable lung at hand, the PaO2/FIO2 ratio may vary greatly.
This is well illustrated in a comparative study published in 2005 by the American Journal of Respiratory and Critical Care Medicine, with Salvatore Grasso as lead author, and performed in ARDS patients.
In patients with no significant recruitable lung (non-recruiter), higher PEEP had no effect on the PaO2/FIO2 ratio.
In recruiters in contrast, PEEP often causes this ratio to increase leading to a downgrade in ARDS severity. In addition, other patients may not meet the ARDS definition any more (ratio above 300 on high PEEP).
Secondly, the presence of distinct ARDS populations (recruiters and non-recruiters), undermines and discredits the use of FIO2 tables proposed by some to set the PEEP level.
Indeed, in patients with a similar large shunt, but with a marked difference in the amount of recruitable lung (and therefore, response to PEEP), such tables would lead physicians to choose a high PEEP (persistent high FIO2 requirement) in the non-recruiters, who are the least likely to benefit from PEEP.
Comparatively, such tables lead to choosing a lower PEEP in the recruiters than in the non-recruiters, given that the response to PEEP allows reducing the FIO2.
Everything else being equal, setting the PEEP based on such table:
- it puts the non-recruitable patients at risk of volutrauma (high PEEP no recruitment)
- it puts the recruitable patients at risk for atelectrauma from tidal opening and collapse (PEEP level too low to prevent cyclical alveolar collapse)
We believe that such tables lack sound physiological rationale and validation; thus, it should not be used to guide the choice of PEEP.
It is also worth mentioning that in the first ARDS net trial, which compared low versus high tidal volume, the strategy associated with the highest PaO2/FIO2 ratio at the onset of the study, was the high-tidal volume approach.
The latter was associated with the worse outcome.
It is, thus, important not to infer from the ARDS severity definition that the best strategy can be identified by simply looking at its impact on the PaO2/FIO2 ratio, which does not appear to be a reliable outcome surrogate.
As a final word, venous admixture correlates with the non-inflated tissue mass and lung compliance, with the size of the normally aerated lung or “baby lung.”
Any obvious discrepancy between the extent of lung infiltrates and/or respiratory mechanics on one end and the degree of venous admixture (e.g., severe shunt, with unexpected low amount of infiltrates and/or essentially unaltered respiratory system compliance) should raise the possibility that other factors might be contributing to low arterial oxygen content, as we shall now discuss.
Perfusion Factors
In addition to taking into account the number of alveolar units not contributing to gas exchange, one also has to consider the factors that regulate their perfusion.
The reduced blood flow through non-aerated alveoli in response to alveolar hypoxia can vary significantly because of the presence or absence of factors, which have the potential to either enhance or blunt the hypoxic vasoconstriction response.
For instance, sepsis, alkalemia, a high cardiac output during positive pressure ventilation (but not during spontaneous breathing or medications, such as intravenous vasodilators) tend to blunt the hypoxic response and increase the degree of venous admixture.
In contrast, inhaled vasodilators or positioning with the good lung down or prone help to redistribute blood flow to the aerated lung and thus, to reduce venous admixture.
Finally, the application of excessive airway pressure on an aerated compliant lung (when the other one is extensively consolidated and non-compliant) results in:
- alveolar vessel compression in the good lung
- redistribution of blood flow to the bad one
- increasing shunt
The wide range of correlations reported (r = 0.5 to 0.9) between the PaO2/FIO2 ratio and degree of shunt points toward PaO2/FIO2 ratio modulator factors other than shunt alone, as we shall now discuss.
Extra Pulmonary Factors
The extra pulmonary factors are these:
- mixed venous oxygen content
- cardiac output
- cardiac shunt
As discussed above, mixed venous O2 content together with the percentage of cardiac output that bypasses aerated lung units (venous admixture) are key determinants of the PaO2 and arterial O2 saturation and content in patients with ARDS.
As the mixed venous O2 content and saturation decrease, so does the arterial PaO2 in the setting of shunt physiology.
It follows that any primary increase in tissue consumption (VO2) or reduction in tissue oxygen delivery (DO2) associated with a compensatory increase in O2 extraction may cause a drop in the mixed venous PO2 and O2 content and saturation and therefore, in the PaO2.
This effect is trivial for a small pulmonary shunt, but significant for a large one.
In ARDS patients, a drop in cardiac output is not an uncommon cause of decreased PaO2. This should be suspected in all patients, who developed a drop in O2 saturation, with unchanged respiratory system mechanics, particularly if it is associated with hypotension.
The effect of an increase in CO on the mixed venous O2 is more complex as the resulting increase in the mixed venous PO2 and O2 content and saturation may be offset by the greater shunt associated with the higher CO.
It is important to consider the cardiac output and the relationship between O2 delivery and demand as a potential cause of a change in PaO2 in ARDS.
For instance, if a patient meets severe ARDS criteria mainly due to a low CO and reduced mixed venous O2 saturation as opposed to a large shunt, the priority would be to restore an adequate hemodynamic and not to embark on recruitment maneuvers or extracorporeal membrane oxygenation.
Not distinguishing the different mechanisms and the cause for reaching a PaO2/FIO2 less than 100 may thus lead to very inappropriate intervention (increasing the PEEP in a patient with low CO due to decompensated cor pulmonale- the Latin for “pulmonary heart”).
It is also worth remembering that cor pulmonale (which has been reported to be present in approximately 25% of ARDS patients) should be systematically searched for in this setting.
Finally, it is also important to remember that approximately 15% of patients with ARDS may also have a cardiac shunt from right to left through a patent foramen ovale (PFO).
An echocardiogram with a bubble study should thus be obtained whenever a low PaO2/FIO2 ratio cannot be clearly explained by the extent of the non-aerated alveolar process alone, a low-mixed venous O2 saturation or circumstances known to be associated with impaired hypoxic vasoconstriction.
Worsening gas exchange when PEEP is dialed up not only can be an important clue to the presence of a right to left shunt through a PFO, but also can be seen in the presence of a decompensated cor pulmonale or hypovolemia.
Those three possibilities are usually easy to differentiate from each other by a bedside echocardiogram.
Physicians And Fractional Inspired Oxygen Factors
Unintended physicians’ contributions to the PaO2/FIO2 ratio are often under-appreciated.
This is the consequence of variable practice and recommendation regarding which PaO2 to target in ARDS. In the ARDS net trials, the explicit targets were 55 to 80 millimeters of mercury (mm Hg) for the PaO2 and 88 to 95 for the arterial O2 saturation.
Let us consider the implication of those targets in a patient with a 30% shunt.
If one targets a PaO2 of 60 mm Hg, the FIO2 required to achieve that target would be 0.5 and the corresponding PaO2/FIO2 would be 120 (moderate ARDS).
If one targets a PaO2 of 80 mm Hg, the required FIO2 would be 1 and the resulting PaO2/FIO2 would be 80 (severe ARDS).
In other words, depending on the targeted PaO2, the apparent ARDS severity may change.
Finally, it is important to stress that varying the FIO2 has different effects on the PaO2/FIO2 ratio depending on the degree of:
- intrapulmonary shunt
- arterio-venous differences
- PaCO2
- respiratory quotient
- hemoglobin under conditions of constant metabolism and ventilation/perfusion abnormality
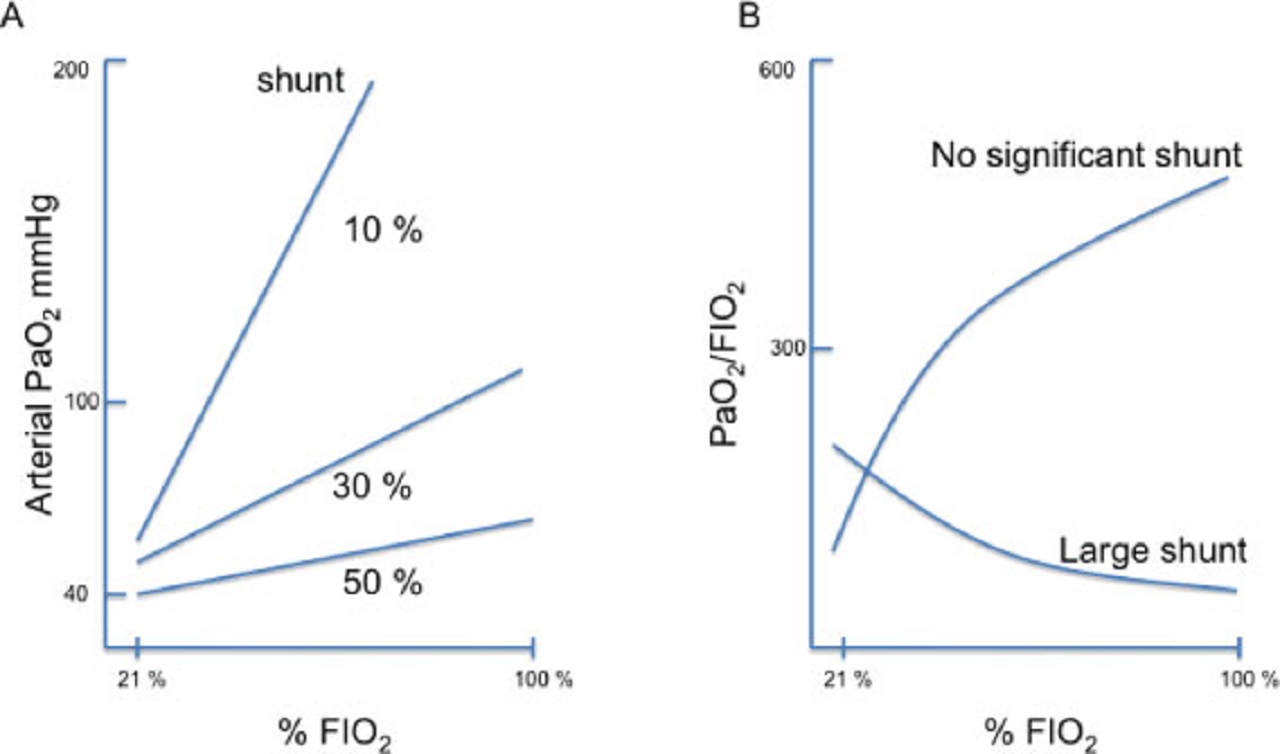
For instance, increasing the FIO2 causes the PaO2/FIO2 ratio to rise if the intrapulmonary shunt is small, but to drop if the shunt is large.
Conclusion
As implied by the ARDS definition, the PaO2/FIO2 ratio should ideally closely reflect the degree of lung injury and extent of alveolar flooding/collapse.
Although there is clearly an association between this ratio and ARDS severity at least when the definition is applied to a population, the reality at the bedside is more complex, particularly if this ratio is calculated without standardizing the levels of FIO2 (e.g., measurement at an FIO2 of 1 avoid the problem of the variable impact of the FIO2 on the ratio) and of PEEP, given that some patients are recruiters and others are not.
As discussed above, the PaO2/FIO2 ratio does not linearly track the degree of lung injury (severity) and may change for reasons that are completely independent of the lungs (e.g., a change in CO and mixed venous O2).
Simply turning up the FIO2 or PEEP knob when PaO2/FIO2 decreases and assuming that in every patient this ratio more or less linearly reflects ARDS severity is a potentially harmful approach.
Interpreting this ratio at the bedside is not that simple and requires:
- a good understanding of cardiopulmonary physiology
- a sound clinical judgment
- a thoughtful approach
A correct interpretation may require obtaining and/or reviewing the following data:
1. an arterial and venous blood gas to confirm the pulse oximetry reading and assess the venous O2 saturation
2. recent ventilator setting changes particularly to PEEP, FIO2 and tidal volume
3. the chest X-ray or computed tomography and respiratory mechanic measurements to assess the alveolar flooding or collapse and the size of the “baby lung” (to look for unaccounted discrepancy between those parameters and the degree of shunt present)
4. a hemodynamic/cardiac evaluation by performing a point-of-care ultrasound to assess for the presence of cor pulmonale and cardiac shunt
5. the conditions that have the potential to affect hypoxic vasoconstriction.
We need a more individualized approach of hypoxic respiratory failure and ARDS.
It is questionable that the new Berlin ARDS definition was the most required change to our approach of ARDS.
One could argue that our patients could be better off, if we had moved away from trying to find commonality between very different conditions as the old and new ARDS definitions do.
The “one size fits all” approach tried for many years has not led to substantial progress.
It may be high time for a different strategy, and for the meantime, it may also be wise to use physiology as a compass to avoid the obvious mistakes associated with a cookbook approach.
We also believe that studying pressure of arterial oxygen to fractional inspired oxygen concentration further is important to optimize the management of patients with ARDS.

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles