Renal Sympathetic Denervation - How Does It Work, Benefits, And Techniques
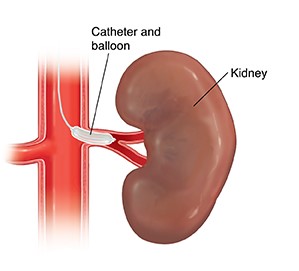
Renal sympathetic denervation is a procedure that uses catheter-based techniques to ablate particular parts of the renal artery nerves to reduce sympathetic nerve activity and blood pressure.
Author:Suleman ShahReviewer:Han JuJul 17, 202355.7K Shares773.9K Views
Renal sympathetic denervationis a procedure that uses catheter-based techniques to ablate particular parts of the renal artery nerves to reduce sympathetic nerve activity and blood pressure. Several trials utilizing renal artery denervation in individuals with resistant hypertension have previously proven effective.
Resistant essential hypertension offers a significant therapeutic challenge since it does not respond to typical treatment regimens. Catheter-based sympathetic renal denervation therapyis an interesting and promising new therapeutic method that has the potential to change the present treatment paradigm in this patient population.
Renal Denervation For Resistant Hypertension
Hypertension is a global public healthcrisis, accounting for 47% of all ischemic heart disease globally and 92 million disease-adjusted lifeyears. While the illness burden in developed countries continues to climb, recent trends reveal a fast increasing prevalence in emerging countries. The core of essential hypertension therapy is a mix of lifestyle changes and pharmaceutical treatments. Although these interventions are usually helpful, a sizable group of individuals whose blood pressure does not react appropriately to them. It is believed that impaired renal excretory function plays a crucial role in hypertension onset, progression, and maintenance.
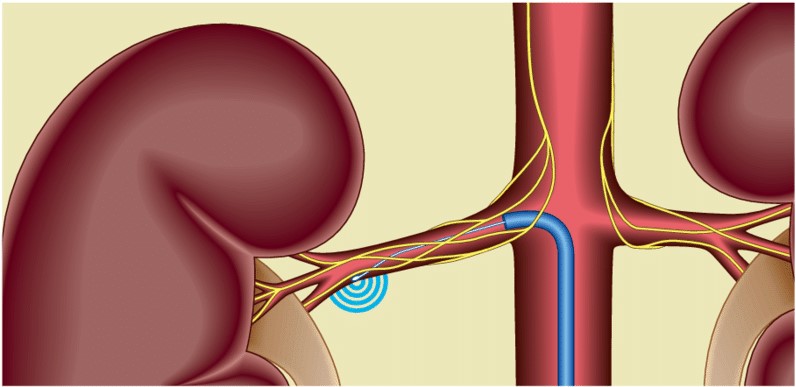
The kidney plays a vital role in salt and water homeostasis, which is essential for arterial pressure management. In individuals with essential hypertension, increased sympathetic activity increases norepinephrine spillover rates from the kidneys. In contrast to the first crude surgical procedure, a more focused renal sympathetic denervation approach employing catheter-based technologyshowed promise for the treatment of resistant hypertension, with minimal side effects. As a result, reducing both afferent and efferent sympathetic activity with renal sympathetic denervation is a sensible strategy for treating hypertension.
Renal Sympathetic Denervation Current Devices
Symplicity
The Symplicity (Medtronic Inc., CA, USA) catheter, intended to transfer radiofrequency radiation through the renal artery wall to accomplish renal denervation, has had the most clinical experience to date. It is presently the only commercially available device with evidence of clinical use from randomized controlled trials. Before operating on the contralateral artery, radiological energy alternating at radiofrequency is administered for 2 minutes and repeated four to six times in a helical fashion. If each side received six treatments, the total treatment duration would be 24 minutes. According to a short trial, renal denervation with the Symplicity catheter showed an excellent safety profile and resulted in a considerable and persistent office BP drop of 27/17 mmHg after 12 months.
Symplicity HTN-2 is the biggest prospective, randomized experiment to date. This research comprised 106 people who were randomly assigned to either renal denervation or a control group, with inclusion criteria comparable to the previous trial. Symplicity HTN-3 is randomized, single-blind research that compares renal denervation against a sham operation. Its enrollment is complete, and the primary findings were due in 2014. The main endpoints are changes in office systolic blood pressure from baseline to 6 months and significant adverse events from baseline to 1 month. According to a recent press statement from Medtronic, Inc., Symplicity HTN-3 did not meet its crucial effectiveness end objective despite no safety issues.
EnligHTN System
Among the various firms producing renal denervation devices, four have received the EU CE mark: EnligHTNTM (St Jude Medical, MN, USA), VessixTM Vascular V2 (Boston Scientific, MA, USA), OneShotTM (Covidien, CA, USA), and PARADISE® (ReCor Medical, CA, USA). Despite the lack of randomized trial data for any of these devices, early first-in-human studies have shown promise. Although the drop in-office blood pressure after six months seems comparable to that reported in the early Symplicity trials, these studies also included ambulatory blood pressure data, though official reporting is still pending.
The EnligHTN system is 8 F-compatible and consists of four monopolar electrodes on an extensible basket with a deflectable tip linked to an radiofrequency generator. On the skin is placed a typical dispersive electrode (grounding pad). The EnligHTN I research looked at the safety and BP decrease of 46 people who had an office systolic BP of 160 mmHg (or 150 mmHg if they had diabetes) and were using three or more antihypertensive drugs. The mean office BP decrease at six months was 26/10 mmHg.
OneShot System
The OneShot Device is a balloon-mounted 9 F-compatible system with a helical silver monopolar electrode coupled to an radiofrequency generator. During ablation, the balloon is inflated to nominal size using normal saline at 1 Atm pressure, and saline seeps through micropores, irrigating, chilling, and minimizing injury to non-target tissue. Treatment duration is usually 2 minutes on each side. At 12 months, an early feasibility study of nine patients revealed a decrease in office BP of 31/10 mmHg. A randomized controlled study is now underway.
Vessix V2 System
The Vessix V2 is a balloon-delivered device with bipolar radiofrequency electrodes installed on the balloon in a helical configuration. All electrodes supply energy at the same time. Typically, 30 seconds are required to give ablative energy on each side, resulting in a shorter procedure than the Symplicity system. The preliminary findings of a 120 patient feasibility trial showed a 27/12 mmHg office BP decrease at six months.
Renal Sympathetic Denervation Future Devices
The Symplicity Spyral Multi-Electrode renal denervation catheter (Medtronic, Inc.) delivers radiofrequency energy through a highly conformable catheter using four electrodes. TIVUS (Cardiosonic, Tel Aviv, Israel) is a preclinical ultrasound device that uses high-intensity ultrasound delivered endoluminal. The Kona Medical System (Campbell, California, USA) employs low-intensity focused ultrasound directed from an external source.
Furthermore, typical electrophysiological microcatheters for renal denervation treatments have been investigated. Concerns that heat created at the tissue-electrode interface during radiofrequency energy administration may cause surface harm but restrict the depth of the lesion while producing a nidus for char development on the catheter are theoretical benefits of using this technology. ThermoCool (Biosense Webster, CA, USA) and Mariner (Medtronic, Inc.) tested devices with encouraging results.
Locally administered autonomic nerve-blocking medications are another approach for establishing renal denervation. The Bullfrog Microinfusion catheter (Mercator MedSystems, Inc., CA, USA) is a balloon-sheathed microneedle catheter. Localized sympathectomy is accomplished by delivering guanethidine via the microneedle after the balloon has been stabilized by saline inflation. Early research in pig models revealed effective medication administration and a considerable decrease in renal norepinephrine output.
Other innovative device technologies are in the works, and current catheter systems are being upgraded. Many systems are projected to become 6 F guide-compatible and capable of accessing the renal arteries through a radial approach shortly, which should lessen vascular access site-related difficulties. Noninvasive denervation techniques may also be available, such as externally administered ultrasound.
Renal Sympathetic Denervation Current Status
Renal sympathetic denervation is not currently commercially accessible in the United States, with the technology reserved for exploratory usage in clinical studies. As a result, there are no official consensus guidelines. On the other hand, the European Society of Hypertension and the European Society of Cardiology have issued policy statements recognizing that renal sympathetic denervation is an effective treatment when used on carefully chosen patients with documented resistant hypertension. Both organizations acknowledged several unsolved problems and emphasized the need for expert input on patient selection, cost-effectiveness, and assessing the benefits/limitations of present renal sympathetic denervation technology.
- Patient Selection:A hypertension specialist at a specialized facility should make the appropriate patient selection. There is no proof that renal sympathetic denervation is a replacement for pharmacotherapy. Thus optimal medical treatment is a must. renal sympathetic denervation requires physically right renal arteries. Current technology necessitates a suitable length and caliber before the first vascular bifurcation, usually a minimum of 20 mm in length and 4 mm in diameter. Although there are anecdotal cases of simultaneous denervation and renal artery stenting in individuals with the renovascular disease, the arteries must also be free of severe stenosis. Some of the devices can denervate accessory renal arteries. However, it is unclear if denervating smaller channels provide therapeutic value. Preprocedural renal anatomy is best examined with computed tomography or MRI renal angiography. In people with moderate-to-severe renal impairment, for example, ultrasound paired with invasive angiography at the time of denervation may serve as an initial screening method.
- Economic viability:The scarcity of clinical trial data and the short patient follow-up period cost-effectiveness restrict cost-effectiveness analyses. Despite the initial capital cost of the generator and the accompanying device and procedure expenditures, renal sympathetic denervation looks to be a very cost-effective technique on a per-patient basis. According to a recent study, the discounted lifetime additional cost-effectiveness ratio was $3071 per quality-adjusted life-year. renal sympathetic denervation was less expensive for 160 and 172 mmHg systolic blood pressure. While renal sympathetic denervation has an extra cost at the time of treatment, if the BP drop is maintained over time, it will become more cost-efficient.
Renal Sympathetic Denervation Benefits
Besides hypertension, excessive sympathetic activity plays a key role in various other illnesses. Renal sympathetic denervation may have a therapeutic function in treating diabetes, heart failure, chronic renal disease, and arrhythmias while being speculative and unproven.
Managing Glucose
Sympathetic nerve activity is crucial in the development of insulin resistance and diabetes. A recent short study looked at the effect of renal sympathetic denervation on glucose metabolism in people with resistant hypertension. Treatment-resistant hypertension patients (n = 37) who received bilateral renal sympathetic denervation were compared to control patients (n = 13). At 3 months of follow-up, those who had renal sympathetic denervation experienced substantial decreases in fasting glucose levels (118-108 mg/dl; p = 0.039) and basal insulin needs (20.8 3.0 to 9.3 2.5 IU/ml; p = 0.006). Similarly, 10 individuals with resistant hypertension and obstructive sleep apnea improved glucose tolerance after renal sympathetic denervation.
Arrhythmias Of Heart
Because autonomic tone modulates chronotropy, dromotropy, the sinus node, and atrioventricular conduction, renal sympathetic denervation may be effective in arrhythmia therapy. Renal sympathetic denervation has been proven in humans to lower heart rate while increasing PR interval. Renal sympathetic denervation has been found in animal experiments to enhance rate regulation and reduce the incidence of atrial fibrillation. In a recent modest hypothesis-generating research, 27 patients with atrial fibrillation and hypertension undergoing pulmonary vein isolation were randomly assigned to receive renal sympathetic denervation (n = 13) or a control (n = 14). At 12 months, individuals who received both pulmonary vein isolation and renal sympathetic denervation had a considerably decreased risk of recurrence of atrial fibrillation. Renal sympathetic denervation has also been used effectively in two individuals with nonobstructive hypertrophic cardiomyopathy and dilated cardiomyopathy for ventricular tachyarrhythmia storm. Both participants significantly improved ventricular tachyarrhythmia frequency after renal sympathetic denervation. More experience in both environments is required before making strong conclusions.
Heart Disease
Pharmacological betablockade has been demonstrated to improve cardiovascular morbidity and mortality in systolic heart failure, likely due to sympathetic nerve activity regulation. Recent research on renal sympathetic denervation in this setting reveals that after 6 months, this intervention may improve symptoms and exercise tolerance. Similarly, renal sympathetic denervation has been demonstrated to decrease LV mass and improve diastolic dysfunction in patients with resistant hypertension. This finding has fueled the hypothesis that renal sympathetic denervation may enhance outcomes for heart failure patients with a maintained LV ejection fraction. More research on the effect of renal sympathetic denervation in heart failure with normal systolic function is now being conducted.
Unresolved Issues
Although there is mounting evidence that renal sympathetic denervation lowers blood pressure, no impact on morbidity or death has been established. No studies show renal sympathetic denervation affects myocardial infarction, stroke, heart failure, renal failure, or mortality. Some apparent improvements with renal sympathetic denervation might be due to a placebo effect. Due to the presence of a doctor in the consultation room, the 'alert response' and 'white-coat' effect may be more pronounced during visit one. In contrast, other healthcare professionals may take follow-up measures. The primary safety end goal combines all-cause mortality, vascular complications, and procedure-related complications.
There seems to be a nonresponse rate to renal sympathetic denervation between 10% and 30%. Methods for determining if nonresponse is due to partial denervation may be required.
People Also Ask
Is Renal Denervation Permanent?
Renal denervation is permanent. Medication for high blood pressure may be discontinued.
Is Renal Denervation Effective?
Renal denervation, a minimally invasive technique, is a potential new non-drug therapeutic option that may successfully manage blood pressure in people with resistant hypertension, according to growing research.
What Is Renal Sympathetic Nerve Activity?
Pathophysiological diseases such as hypertension and chronic and end-stage renal illness typically increase renal sympathetic nerve activity (RSNA). Increased RSNA elevates blood pressure and may lead to renal function impairment.
What Does Renal Denervation Do?
Renal denervation (RDN) is a minimally invasive treatment used to treat resistant hypertension. Radiofrequency ablation is used in the operation to burn the nerves in the renal arteries. This technique reduces nerve activity, which lowers blood pressure.
Conclusion
The preliminary results of catheter-based renal sympathetic denervation research have been encouraging. However, randomized controlled trial data, including outcome measures, are needed to analyze the effectiveness of renal sympathetic denervation further and clarify its role in modern practice. Renal sympathetic denervation has the potential to be a valuable supplement to the best medical therapy for the treatment of resistant hypertension.
Although the current data is inconclusive due to several methodological flaws, ongoing research, including randomized trials incorporating a sham procedure, will help define the efficacy of renal sympathetic denervation compared to best medical therapy and the role of this procedure in contemporary practice. Although renal sympathetic denervation is now being studied mainly to lower blood pressure, it may be used in various applications where sympathetic nervous system overactivity is deleterious. This includes illnesses like heart failure, when renal sympathetic denervation may be used to supplement existing prescription treatment.

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles