The Human Retina - Intrinsically Photosensitive Ganglion Cells In The Retina
In recent years, there has been a rise in public awareness of the impacts of light on health, especially the harmful ones that might result from improper timing of light delivery. Nighttime lighting, also known as "light pollution," is becoming increasingly hazardous to both the environment and human health. Even very small amounts of light from phones, tablets, and other devices that give off light can make it hard to sleep.
Author:Suleman ShahReviewer:Han JuJul 29, 202211.6K Shares777.6K Views
In recent years, there has been a rise in public awareness of the impacts of light on health, especially the harmful ones that might result from improper timing of light delivery.
Nighttime lighting, also known as "light pollution," is becoming increasingly hazardous to both the environment and human health. Even very small amounts of light from phones, tablets, and other devices that give off light can make it hard to sleep.
As insufficient lighting can be harmful to health, optimal lighting can be a straightforward, affordable population-level intervention to improve health. For instance, if light is given at the right time and in the right amount, it can improve the quality of lifein nursing homes and help people think more clearly at work and in school.
Along with the well-known rods and cones, which are photoreceptors that help us see, there is a third type of cell in the human retinathat controls both the good and bad effects of light.
These cells are a subpopulation of retinal ganglion cells (RGCs), which are photosensitive due to the photopigment melanopsin that they produce.
They have been called either photosensitive, intrinsically photosensitive retinal ganglion cells (pRGCs, ipRGCs), or melanopsin-expressing retinal ganglion cells (mRGCs). This depends on whether the studies are looking at how they react to light or whether they have melanopsin.
The so-called "non-visual" or "non-image-forming" responses to light are mostly mediated by ipRGCs.
Some of these reactions are getting our internal clock to match the natural day-night cycle, controlling our sleep-wake cycle, adjusting our pupillary reflex to light (PLR), and changing our mood.
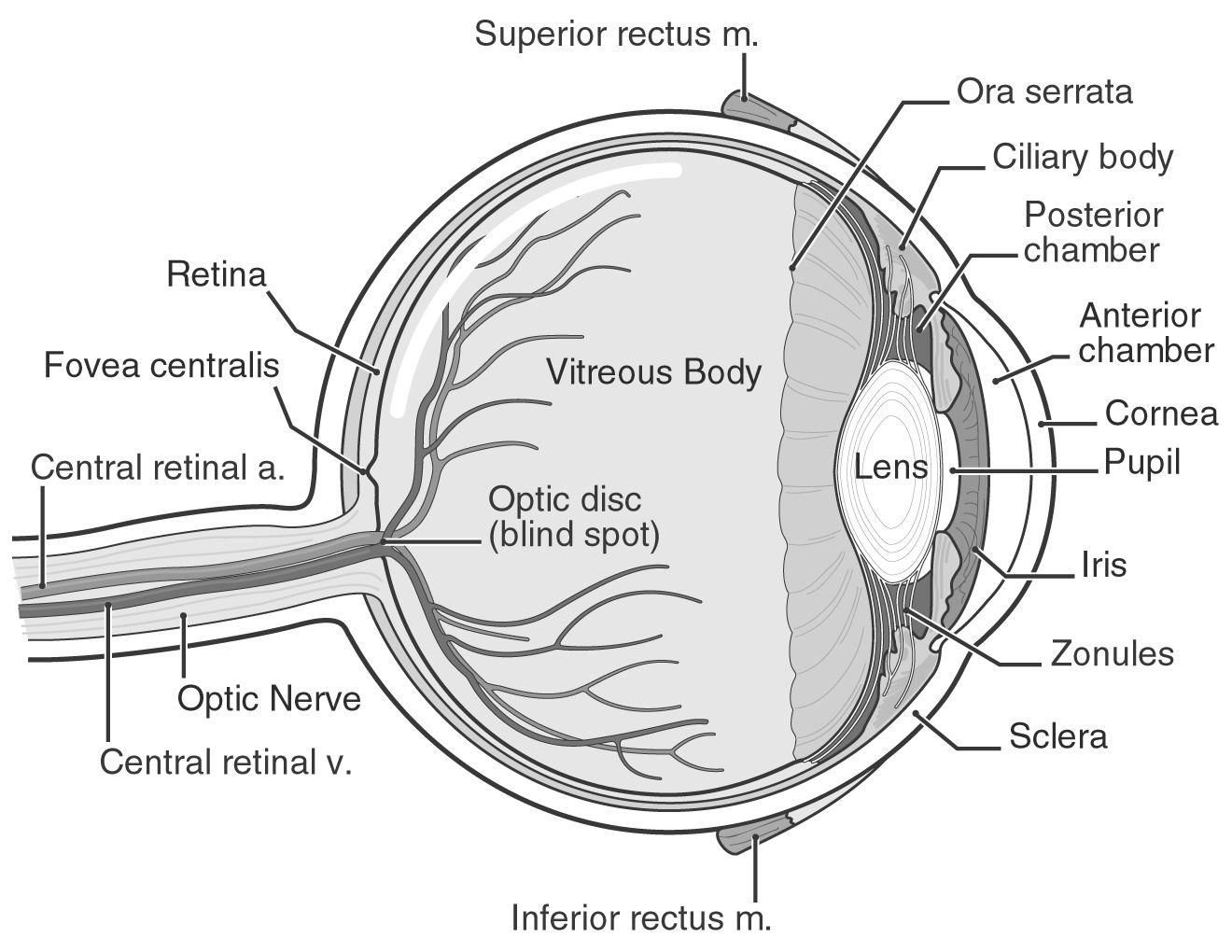
What Is The Retina Of The Eye?
The retina is a crucial link between the light that enters your eyes and the pictures you perceive. Your retina has specialized cells that respond to light and send information to your brain so you can see the world around you. If you detect any changes in your eyes, contact your doctor straight away.
Light that enters your eye is converted by the retina into electrical impulses that your optic nerve transmits to your brain, which produces the pictures you see. It's an important aspect of your vision.
The layer at the very back of your eyeball is called the retina.

The Retina | What is the Retina and What is its Function?
What Kind Of Material Makes The Retina?
The macula and the peripheral retina are the two components that make up the retina. The majority of what you are currently looking at is processed by the macula, which is in the middle of your retina. The peripheral retina fills in the image in the periphery of your field of vision (your peripheral vision). For instance, if you're seated across from a friend at a table, your peripheral retina allows you to view the remainder of the room on either side of them while your macula aids in seeing their face.
There are several cell kinds in the retina. Your brain can interpret the electrical signal that photoreceptors create from light as pictures. Photoreceptors called rods make it possible to see at night and in low light. Most of your normal eyesight is composed of cones, which process color. Together, these sorts of cells provide a clear, accurate representation of what you are seeing.
How Does The Retina Aid Eye Activity?
Your retina functions as a translator for your eye. When light strikes it, your retina transforms it into a signal that your brain can interpret.
Your eye may still work without a retina or with a damaged retina (it would still take in light), but your brain wouldn't get all the information it needed to produce pictures.
Your eyesight might get worse if anything damages your retina. Therefore, if your eyes suddenly change, you should see your healthcare practitioner as soon as possible.
Human IpRGCs Comprise Several Morphological Subtypes
Melanopsin was discovered in the mouse retina after being discovered in the human retina. Some RGCs in the ganglion cell layer and the inner nuclear cell layer made melanopsin.
Melanopsin-expressing cells have a unique shape with two to four dendritic processes. Melanopsin immunoreactivity is seen in somatic cells, neural processes, and cytoplasmic membranes. Human retinas have melanopsin-positive cones.
Recent studies have examined the morphology of ipRGCs in donors of human retinas. In humans, the number of ipRGCs ranges from ~4,000 to more than 7,000, but it's quite small (0.4–1.5%) compared to the 1.07 million ganglion cells in the retina.
M1 types of mice have dendrites in the outer sublamina of the inner plexiform layer (IPL), whereas M2 mice have dendrites in the inner sublamina. Fovea lacks ipRGCs.
ipRGCs are most prevalent in the peri-foveal area (~15–40 cells/mm2) and fall to <5 cells/mm2 at 10 mm eccentricity and beyond; they track the reduction in RGC density from the center to the periphery of the retina. Specific research has found other types of ipRGCs with different shapes, such as M3, M4, normal M1, giant M1, displaced M1 (dM1), and huge dM1.
In humans but not in mice, dM1 dominates M1. These morphological investigations relied on melanopsin immunostaining, which fails to identify all ipRGCs in mice. This shows an underestimation of ipRGCs and a possible bias in subtype distribution.
IpRGCs Brain Targets
Mapping ipRGC brain projections helped identify their many roles. In mice, ipRGCs convey light information to more than a dozen brain regions, including nuclei implicated in circadian rhythms [suprachiasmatic nucleus (SCN), intergeniculate leaflet (IGL)], sleep and wake regulation [in the hypothalamus, the ventrolateral preoptic area (VLPO) and lateral hypothalamus (LH)], PLR control [olivary pretectal nucleus (OPN) (peri Habenula). The dorsal lateral geniculate nucleus (dLGN) and the superior colliculus (SC) are also targeted.
In humans, ipRGC projections cannot be explored using tracers or genetically encoded labels. In this study, Hannibal and colleagues used the knowledge that pituitary adenylate-cyclase-activating polypeptide (PACAP) is a marker for retinohypothalamic tract (RHT) projections to the SCN in mice and humans to define ipRGC potential SCN projections.
Two human donors had a dense terminal field of PACAP-positive nerve fibers in the SCN retina recipient zone (ventral region) (while no PACAP-immunoreactive cell bodies were found in the SCN). Fibers from the optic chiasma were near to VIP-containing neurons in the ventral SCN.
Since humans cannot employ tracers, investigations into non-human primates are needed to map ipRGC central projections. Classical retrograde tracing in macaques revealed these locations as ipRGC targets.
Macaque ipRGC projections to the SCN were validated by immunohistochemical labeling of PACAP and an anterograde tracer (Cholera Toxin Fragment B). The pregeniculate nucleus [which is assumed to correlate with the rodent IGL], the OPN, the optic tract nucleus, the brachium of the SC, and the SC project to the LGN were identified.
In the macaque, ipRGC projections to the dLGN come from inner and outer stratifying melanopsin cells (therefore possibly from all ipRGC subtypes), but in mice, the bulk of melanopsin ganglion cell innervation of the dLGN seems to be given by inner stratifying cells [non-M1 cells]. The significance of ipRGCs in primate vision remains unclear.
In mice, ipRGC terminals are located in the VLPO, LH, anterior hypothalamic nucleus, ventral subparaventricular zone, and peri-supraoptic nucleus. Primate retinas project to hypothalamic nuclei.
It is uncertain whether these forecasts incorporate ipRGCs. These nuclei regulate physiology by controlling sleep, hunger, and thermoregulation, among other things.
2-Minute Neuroscience: The Retina
Human IpRGC Functional Properties And Diversity
Weinstein et al. reported the first direct RGC EP recording. This research assessed photopic RGC spectral sensitivity (555 nm).
Until recently, retinal recordings were anecdotal. There have been as many peer-reviewed and non-peer-reviewed preprint manuscripts on the physiology of the human retina in the last two years as there were in the 50 years before that.
Only one piece of research has been intended to capture human RGCs' innate sensitivity, light responses, and functional variety. Overall, pharmacologically separated human ipRGC responses look comparable to mice and macaques. Human ipRGCs' light responses are delayed, persistent, and don't end quickly after the light is off.
ipRGC responses are slower than rod and cone-driven responses (<100 ms). Opsinamide inhibits human ipRGCs' intrinsic photoresponses reversibly. Mure et al. observed that ipRGCs have limited intrinsic sensitivity; they don't react to light below photopic level, even after dark adaptation.
Their spectral sensitivity peaked in the blue area (~460 nm), differing from human rods and cones but near to mice and macaque melanopsin peaks and human melanopsin produced in HEK293 cells. This conclusion fits with the fact that ipRGCs are involved in non-visual human light responses like melatonin peak suppression at night, PLR, non-cone/non-rod visual awareness, cognition, and heart rate regulation.
Parameters and time courses imply that human ipRGCs are functionally divided. Mure et al. identified three ipRGC subtypes with distinct kinetics and light sensitivity.
Type 1 ipRGCs respond to light longer after it's switched off. Less sensitive Type 2 ipRGCs switch off quicker. Type 2 ipRGCs have a greater reaction latency at low irradiation levels.
A third class of ipRGCs reacted solely to exogenous 11-cis retinal. Type 3 cells react aggressively to high irradiance and are extinguished quickly after light off. Overall, Type 1, Type 2, and Type 3 ipRGCs resemble the mice M1, M2, and M4 ipRGCs.
The relationship between human physiological and morphological ipRGC subtypes and mice subtypes is unknown. Mure et al.’s research used a small number of donors; these results must be repeated.
The effort must be pursued to refine the findings and improve the quantity and variety of contributors. Light-induced melatonin suppression in the evening, controlled by ipRGCs, varies 50-fold amongst people. It would be interesting to determine to what degree ipRGCs contribute to light sensitivity variability.
Transcriptome Diversity Of Human IpRGCs
ipRGC gene expression patterns underlie morphological and functional diversity. In mice, all ipRGCs express Brn3b except for the percentage of M1 cells that project to the SCN.
Two molecularly distinct subpopulations of M1 ipRGCs innervate separate brain areas (SCN for M1 Brn3b and OPN for M1 Brn3b+). This new identity dimension is accessible. Single-cell RNA sequencing (scRNAseq) or RNA sequencing (RNAseq) on RGC-enriched samples made it possible to separate the ipRGC subpopulations of mice and primates.
In the macaque and human retina, scRNAseq on CD90+ cells to enrich samples with RGCs (CD90 or Thy1 is an RGC class identifier) permitted distinguishing up to 18 RGC subpopulations. ON and OFF midget RGCs and ON and OFF parasol RGCs make up >80% and ~10% of all RGCs in primate retinas.
The remaining RGC clusters include ~1% of all RGCs. Three RGC clusters in the macaque and two in humans showed measurable quantities of melanopsin. In humans, the authors noted a difference in melanopsin expression levels and hypothesized a correspondence between the cluster expressing the highest level of melanopsin and M1 ipRGCs, which express the highest levels of melanopsin in mice. Other subtypes (M2–M6) would constitute the remaining cluster or be too rare to detect.
Interestingly, ganglion cells are the least preserved retinal cell type across mice and macaques. Unlike conventional RGCs, ipRGCs are well preserved in diversity and distribution.
This may be because nocturnal and diurnal animals monitor different visual signals and have different visual systems. In contrast, non-visual reactions to light, such as the circadian clock, are comparable for most creatures and may use similar cell types.
Integration Of External Input From Photoreceptors
ipRGCs combine extrinsic inputs with their intrinsic photosensitivity, like ordinary RGCs. By comparing the responses of ipRGCs before and after they were given synaptic blockers that stop RGCs from receiving input from outside sources, the outer retina photoreceptor contribution to ipRGC signaling in humans can be seen.
The preparation may impact photoreceptor responses differently. In vitro, without RPE, rods and cones may contribute less. Light activates many RGCs without synaptic blockers.
After incubation with blockers, most RGCs stop receiving rod and cone impulses. ipRGC answers survive but are changed. Response threshold, latency, and amplitude are all greater. Rod and cone responses appear to be ipRGC subtype-specific.
Extrinsic input reduces reaction latencies and thresholds for all ipRGC subtypes. Extrinsic input only increased the sensitivity of Type 2 and 3 ipRGCs. In mice, the contribution of rods and cones to ipRGC responses is inversely related to how sensitive melanopsin is to light. This has a small effect on M1 ipRGC responses, but M2–M5 subtype responses depend more on inputs from outside the eye.
Type 3 ipRGCs appear to depend mainly on rods and cones, similar to M4 ipRGCs. Type 1 ipRGCs get little extrinsic input. M1 ipRGCs, mice homologue of type 1 ipRGCs, photo entrain the clock. This supports the idea that cones may contribute to human clock entrainment but are not needed.
Human and mice cones vary in quantity and peak wavelength sensitivity, suggesting distinct ipRGC input in response to the same light stimulus. Functional differences may exist. Short-wavelength cones and melanopsin inhibit primate PLR but enhance mice PLR.
The subtype-specific contributions of rods and cones may drastically modify ipRGC spectral sensitivity, shifting action spectra from blue to shorter or longer wavelengths.
IpRGCs In Aging And Disease
Several studies have shown that neurodegenerative illnesses accelerate the loss of ipRGCs with age. Aging reduces the amount of ipRGCs and dendritic arborization by 31% in healthy people over 70.
The functional relevance of this drop is disputed. Some data implies that ipRGC response qualities may demonstrate functional compensation by increasing sensitivity and/or firing rate such that no substantial change in ipRGC-dependent response such as PLR is found in older people.
Bright light helps enhance the elderly's decreased circadian rhythm and sleep fragmentation. ipRGC responses in an elderly donor (> 70 years) are slower and shorter.This data shows that aging may change ipRGCs' number and function.
Alzheimer's and Parkinson's disorders (AD and PD) exacerbate aging-related ipRGC depletion. AD and PD patients have 25–30% fewer ipRGCs than age-matched controls, and surviving ipRGCs are dendritic. AD patients' ipRGCs may have impaired neuronal physiology due to protein aggregation.
ipRGC degradation may cause circadian rhythm and sleep disruption in neurodegenerative diseases. Initially more durable than ordinary RGCs, ipRGCs are lost in glaucoma.
In diabetic retinopathy, ipRGC loss corresponds with RGC loss overall. In old age and neurological disorders, ipRGCs diminish histologically.
Some data shows that ipRGCs' function is also changed with age, although whether their intrinsic light response, rod and cone input, and/or subtype abundance are impacted has to be determined.
People Also Ask
What Does The Human Retina Contain?
In the human retina and the retinas of the majority of vertebrates, there are two types of photosensitive cells: rods and cones. The rods are typically considerably thinner than the cones, although both are constructed using the same general framework. The outer segment, which sits on the pigment epithelium, houses the light-sensitive pigment.
Why Is The Human Retina Backwards?
Because the visual cells are orientated such that their sensory ends are pointed away from incoming light, this configuration of the retina is referred to as being inverted in biology. Few mollusks and spiders are the only non-vertebrates that have it, and it is typical of vertebrates.
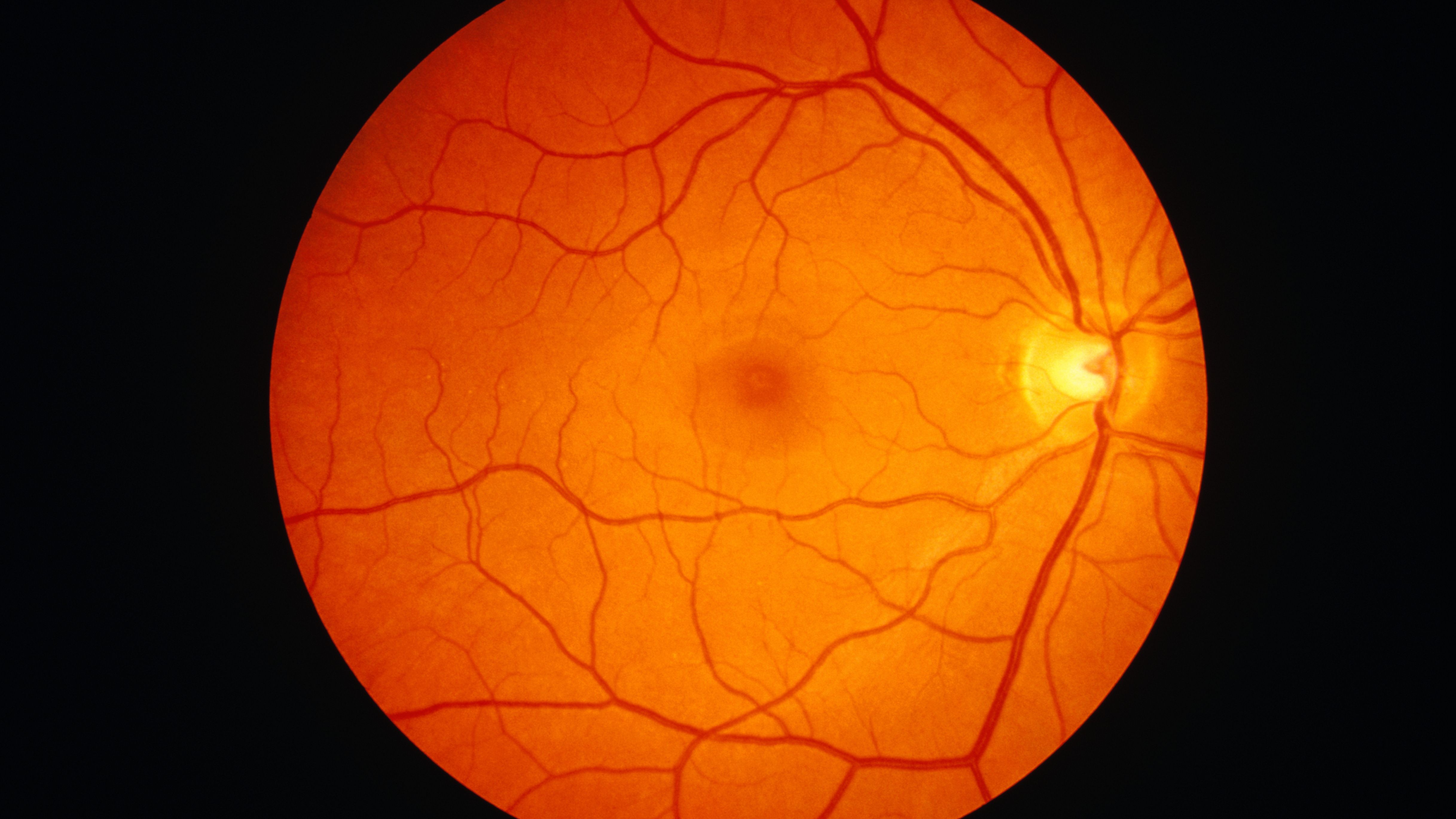
What Color Is The Human Retina?
An inner layer of pigment cells in the retina gives the interior of the eye its orange hue. One cell thick, this pigment layer absorbs incoming light and stops it from dispersing. A clearer perspective is the end outcome.
Is The Human Retina Part Of The Brain?
The retina really develops from neural tissue throughout embryonic development and is joined to the brain via the optic nerve. Light-sensitive photoreceptor cells are only found in one layer of the retina, which is a transparent tissue with many layers.
Conclusion
The variations between mice and primates clearly demonstrate the need to use donors from human retinas in the established models. Non-human primates are still required for certain investigations, such as projector mapping.
They do not, however, provide any advantages over human preparations in termsof ethics or productivity, and as a result, a bigger sample size is not possible. Also, at least for surgical samples, planning and doing the tissue collection in humans and monkeys may take about the same amount of time.
Thus, the variables impacting the preparation's fitness may be managed (hypoxia delay, pH, or nutrients). The creation of new human ex vivo and in vitro models, such as long-term retinal culture or retina organoids, may also be a part of the research of human ipRGC. Some findings are highly positive since retinal organoids exhibit photosensitivity, layering, and cellular variety that somewhat mimics the diversity of the functioning peripheral retina.
Jump to
What Is The Retina Of The Eye?
What Kind Of Material Makes The Retina?
How Does The Retina Aid Eye Activity?
Human IpRGCs Comprise Several Morphological Subtypes
IpRGCs Brain Targets
Human IpRGC Functional Properties And Diversity
Transcriptome Diversity Of Human IpRGCs
Integration Of External Input From Photoreceptors
IpRGCs In Aging And Disease
People Also Ask
Conclusion

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles