Uric Acid In Chronic Kidney Disease - Discussing The Key Insights
A few recent studies have focused on uric acid in chronic kidney disease. They suggested that uric acid may also play a role in heart disease and hypertension.
Author:Suleman ShahReviewer:Han JuAug 12, 2024101.2K Shares1.4M Views
Uric acid is the end product of purine metabolism in humans.
Most mammals possess uricase, an enzyme that converts uric acid to allantoin, a soluble substance that is excreted in the urine.
This enzyme is absent in humans and apes due to mutations in the uricase gene, which rendered it nonfunctional.
High levels of serum urate may occur as a result of:
(1) increased dietary intake of purine sources
(2) increased cell turnover
(3) under-excretion of urate
High intake of fructose has also been associated with high serum urate levels as it causes depletion of ATP and increases the production and release of uric acid.
Approximately two-thirds of uric acid is eliminated by urinary excretion and the remainder is excreted in the feces.
At the glomerular level, urate is freely-filtered and 90% of the filtered urate is reabsorbed.
The main transporters involved in tubular reabsorption are SLC22A12 (URAT1) and SLC2A9 (GLUT9).
There is also evidence of tubular secretion of urate with the involvement of:
| ABCG2 | SLC22A6 (OAT1) |
| SLC17A1 | SLC22A8 (OAT3) |
| SLC17A3 | -- |
Hyperuricemia has traditionally been associated with goutand urolithiasis, which are both caused by deposition of monosodium urate crystals.
Urate, the soluble form of uric acid in the blood, is also an important antioxidant in the plasma.
It reacts with the following by neutralizing their toxic effect:
- hydrogen oxide
- hydroxyl radicals
- nitric oxide (NO) derivatives
Urate also inhibits the degradation of superoxide dismutase, an enzyme with antioxidant activity in the vascular endothelium.
Unfortunately, some studies suggest that urate’s reaction with oxidizing agents may result in the release of active free radicals that may have some deleterious effects.
Furthermore, once uric acid enters the cell, it appears to exert more harmful effects.
Uric acid enters the vascular smooth muscle cells through specific organic anion transporters and activates p38 and Erk ½ and nuclear transcription factors such as nuclear factor kappa B (NF κB) and activator protein-1 (AP-1).
This results in production of:
- growth factors (e.g. platelet-derived growth factor)
- vasoconstrictive substances (angiotensin II and thromboxane A2)
- cytokines
- proinflammatory molecules (C-reactive protein and monocyte-chemoattractant protein-1), which cause inflammationand proliferation of the vascular smooth muscle cells
Uric acid also stimulates the production of interleukin 1B, interleukin 6 and tumor necrosis factor -α, which augments its proinflammatory properties.
Uric acid also:
- causes endothelial dysfunction by means of impaired release of nitric oxide
- promotes vascular thrombosis by triggering platelet adhesion and aggregation
Recently, studies have implicated uric acid as an independent risk factor for:
- hypertension
- cardiovascular disease
- kidney disease.
These associations were thought to be mediated by these biologic effects of uric acid.
The aim of this review was to discuss uric acid in chronic kidney disease.
Uric Acid And Hypertension
As early as the 1800s, a possible association between uric acid and hypertension was proposed.
British physician Frederick Mahomed (1849-1884) noted in 1879 that many of his subjects with essential hypertension came from families with gout. He hypothesized that uric acid might play an integral role in the development of essential hypertension.
In the 1960s to early 1970s, epidemiological studies found that as many as 25-50% of patients with gout had arterial hypertension.
In 2001, an animal model of hyperuricemia was developed, which revitalized the interest in uric acid, hypertension, and renal disease.
In this animal model, rats are given oxonic acid, a uricase inhibitor which causes mild elevation in uric acid levels without causing intrarenal crystal deposition and acute renal failure.
An unexpected finding in this animal model is the development of hypertension after several weeks of mild hyperuricemia.
It was noted that systolic blood pressure correlated with uric acid level and a 1-milligram-per-deciliter (mg/dl) change in uric acid resulted in 30 to 40-millimeter of mercury (mmHg) rise in the systolic blood pressure.
This effect was noted both in the presence of sodium-restricted and normal salt diets.
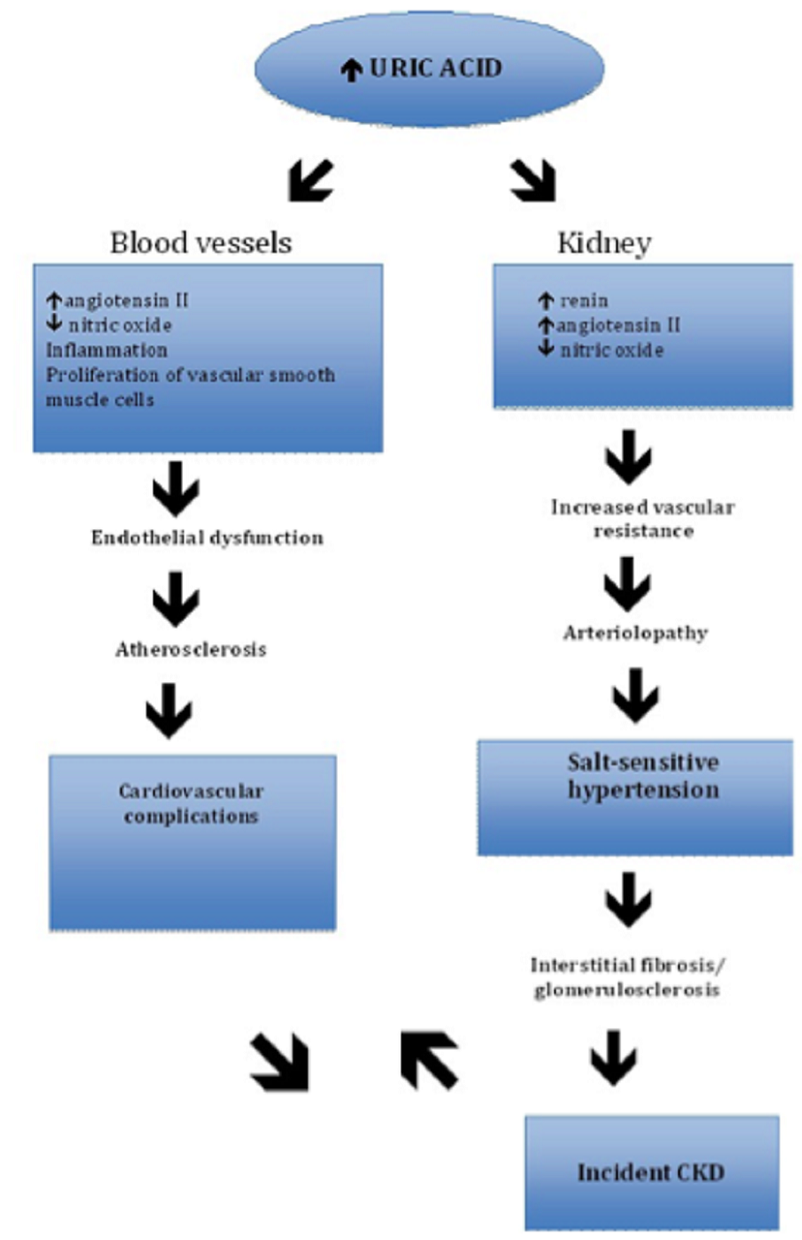
Through various studies that employed this animal model, the mechanisms by which uric acid can cause hypertension were elucidated.
These mechanisms include elevated juxtaglomerular renin expression and reduced nitric oxide synthase, which result in increased renal vascular resistance and increased proximal tubule sodium reabsorption in mice.
Furthermore, it was noted from these animal studies that microvascular renal lesions similar to arteriolosclerosis develop over time in the presence of hyperuricemia.
Once these lesions are present, the subjects develop a salt-sensitive hypertension.
In a study published in 2002 by the American Journal of Physiology-Renal Physiology, the authors, with Marilda Mazzali as lead author, performed a study using this animal model with mild hyperuricemia.
They noted that hyperuricemia induces a primary arteriolopathy in rats, which was not prevented by hydrochlorothiazide despite its ability to lower the blood pressure.
On the other hand, the rats that received enalapril and losartan did not develop arteriolar thickening, which led the authors to conclude that:
- uric acid stimulates vascular smooth muscle in vitro independent of the blood pressure; and
- this effect is mediated by the renin angiotensin aldosterone system
In the human population, several studies also showed that hyperuricemia is associated with an increased risk of hypertension independent of other risk factors, most notably in adults with prehypertension and women.
In their study published in 2003 by the journal Hypertension, Daniel I. Feig and Richard J. Johnson noted that hyperuricemia is more common in adolescents with essential hypertension than secondary hypertension.
Feig and Johnson also that an elevated uric acid level (>5.5 mg/dl) is observed in almost 90% of adolescents with essential hypertension.
Unfortunately, among patients with long-standing hypertension, the results of studies examining the association of hypertension with hyperuricemia have yielded inconsistent results.
In a study published in 1985 by the American Journal of Epidemiology, its authors, with Frederick N. Brand as lead author, noted an inverse relationship between uric acid level and hypertension with increasing age and duration of hypertension.
This suggests that uric acid may be most important in younger populations with early-onset hypertension.
Uric Acid And Cardiovascular Disease
The association of elevated uric acid with risk factors associated with cardiovascular disease has made it difficult to establish a causal relationship between hyperuricemia and cardiovascular disease.
High uric acid levels are often noted in populations at risk for cardiovascular disease such as:
- postmenopausal women
- African Americans
- patients with hypertension, metabolic syndrome, or renal disease
There are several possible mechanisms linking hyperuricemia to cardiovascular disease.
Some investigators proposed that the potential relationship between hyperuricemia and cardiovascular disease could be through direct cardiovascular damage from uric acid’s proinflammatory effect on vascular cells and adipocytes.
Although uric acid is an antioxidant responsible for 60% of the total antioxidant capacity in humans, the rise in serum uric acid in patients with cardiovascular disease might represent a compensatory mechanism to the increased oxidative stress that occurs in this condition.
Endothelial dysfunction, as a consequence of reduced nitric oxide production, is also believed to play a role, particularly in the early development of atherosclerosis.
A systematic review and meta-analysis including 26 studies with 402,997 adults found that hyperuricemia may marginally increase the risk of cardiovascular events independent of other coronary heart disease risk factors (RR 1.09 with 95% CI 1.03-1.16).
Women were also found to have an increased risk for mortality from coronary heart disease in this study.
However, some expert groups have argued that studies indicating uric acid as an independent risk factor did not control for all known risk factors.
The Framingham Heart Study group claimed that uric acid does not have a causal role in the development of coronary artery disease or death from cardiovascular disease.
The authors reported that uric acid’s association with cardiovascular disease is due to its association with other risk factors for cardiovascular disease such as hypertension.
The Atherosclerosis Risk in Communities Study (ARIC) found similar conclusions.
Due to lack of well-powered clinical trials establishing a causal effect, uric acid is not considered a cardiovascular risk factor by major professional societies.
Uric Acid In Chronic Kidney Disease
In the past, the association between hyperuricemia and chronic kidney disease (CKD) was often attributed to decreased uric acid clearance.
Uric acid was used as a marker of renal damage but recent observational studies have raised the possibility that uric acid may have a contributory role in the development of CKD.
Animal studies provided the most robust data supporting the role of uric acid in the development of intrarenal vascular disease and renal injury.
Some of the proposed mechanisms of kidney damage are from uric acid include:
- induction of afferent arteriolopathy
- inflammation
- activation of the renin-angiotensin system
In a study published in 2005 by the journal Kidney International, the authors, with Laura G. Sanchez-Lozada as lead author, rats on a normal salt diet that received oxonic acid showed a two-fold increase in uric acid levels, which was associated with:
- a 10-mmHg rise in the systolic blood pressure
- an 18-mmHg rise in the mean arterial blood pressure
On histologic exam, afferent arteriolar thickening was noted, which led the authors to conclude that hyperuricemia causes arteriolopathy of the preglomerular blood vessels, which impairs the autoregulatory response of the afferent arterioles resulting in glomerular hypertension.
Sanchez-Lozada et al. also believed that the lumen obstruction brought about by arteriolar thickening results in renal hypoperfusion, which stimulates tubulointerstitial inflammation and fibrosis.
The association between hyperuricemia, hypertension, and arteriolar thickening was further supported by the finding that when oxonic acid was administered with allopurinol, both the rise in the blood pressure and the afferent arteriolar thickening were prevented.
In a study published in 202 by the Journal of the American Society of Nephrology, the authors, with Duk-Hee Kang as lead author, utilized the remnant kidney model to investigate the role of uric acid in renal disease progression.
In this study, rats underwent 5/6 nephrectomy and were given oxonic acid for 6 weeks.
It was noted that hyperuricemia resulted in higher blood pressure and intensified renal disease progression with worsening proteinuria and more severe histological lesions such as glomerulosclerosis and interstitial fibrosis.
Hyperuricemia was also associated with increased COX-2 expression and juxtaglomerular renin. Allopurinol, and to a lesser degree benziodarone, reduced the occurrence of these findings.
Unlike animal studies, the results from cross-sectional studies in humans are less straightforward.
A Japanese study, which was performed on 6,403 individuals with normal renal function residing in Okinawa City, concluded that other than gender, serum uric acid is the most significant correlate for developing high serum creatinine over a 2-year follow-up period.
This study further concluded that a serum uric acid level ≥ 8.0 mg/dl is associated with 2.9-fold risk in men and 10-fold risk in women for developing high serum creatinine.
However, baseline uric acid levels did not show a correlation with the change in creatinine clearance as computed by Cockroft-Gault equation, and the authors did not provide an explanation for this.
The same investigators also identified uric acid in women as an independent predictor of renal failure requiring renal replacement therapyduring a 7-year follow-up period, but the results in men failed to reach statistical significance.
The role of uric acid as an independent risk factor for the development of new-onset CKD has been supported by several studies.
In a study published in 2008 by the Journal of the American Society of Nephrology, the authors, with Daniel E. Weiner as lead author, analyzed pooled data from 13,338 participants across two community-based cohort studies, the Atherosclerosis Risks in Communities (ARIC) and the Cardiovascular HealthStudy (CHS).
During the 9-year follow-up period, 5.6% of the study population had incident kidney disease, defined as a decrease in GFR of ≥15 ml/min/1.73 m to a GFR <60 ml/min/1.73 m.
After adjusting for variables (age, gender, diabetes, systolic BP, cardiovascular disease, and hypertension), the investigators found that baseline uric acid level was associated with increased risk for incident kidney disease, with an odds ratio of 1.07 (95% confidence interval 1.01 to 1.14) per 1 mg/dl increase in uric acid.
However, the study did not account for proteinuria and allopurinol use.
Similarly, the result of a community-based cohort study in Vienna showed that a 2 mg/dl increase in serum uric acid is associated with 69% increase in developing new-onset kidney disease.
More recently, the Jerusalem Lipid Research Clinic cohort study also found a similar association between serum uric acid and CKD.
However, the association was not linear with a clear increase in incident CKD, only when serum uric acid levels were:
- >6.5 mg/dl in men
- >5.3 mg/dl in women
This cohort study also found an association between acute kidney injury and uric acid with a 74% increased risk per 1 mg/dl.
In a study published in 2010 by the American Journal of Kidney Diseases, the authors, with Dr. Gianni Bellomo as lead author, performed a prospective cohort study among 900 normotensive subjects
They found similar results implicating serum uric acid as an independent risk factor for decreased GFR after 5 years.
The association between proteinuria and uric acid was the subject of a study published in 2010 by Nephrology Dialysis Transplantation, with Diana I. Jalalas as lead author.
These investigators analyzed data from the Coronary Artery Calcification in Type 1 Diabetes(CACTI) to determine an association between albuminuria and serum uric acid among study participants with Type 1 diabetes and no albuminuria at baseline.
Among the 324 participants included in the analysis, the authors noted that, for every 1 mg/dl increase in serum uric acid, there is an 80% increased risk of developing micro or macroalbuminuria after 6 years of follow-up.
They concluded that serum uric acid is a strong predictor of the development of micro or macroalbuminuria at 6 years, independent of HbA1c and degree of pre-albuminuria (defined as ACR <30 mg/g), in patients with Type 1 diabetes.
While the relationship between uric acid, albuminuria, and more advanced stages of CKD were not examined in this study, this would suggest that there are multiple potential pathways that uric acid may lead to CKD.
Unlike the positive association noted in the cohort studies between uric acid and incident CKD in populations with normal renal function, studies investigating the role of uric acid in patients with established CKD provided varied results.
In a study published in 2009 by the American Journal of Kidney Diseases, the authors, with Magdalena Madero as lead author, analyzed:
- the relationship between baseline uric acid levels and all-cause mortality
- cardiovascular disease mortality
- kidney failure
Among 838 patients with mean GFR of 33 ml/min/1.73 m included in the analysis, uric acid was associated with increased risk for all-cause mortality, primarily through cardiovascular mortality, but not with progression of CKD.
One of the possible explanations cited by the authors is the strict blood pressure control among the study participants assigned to the low blood pressure group, which could have blunted the effect of hyperuricemia on the development of hypertension and kidney failure.
Similar findings were reported by Liu in 2012, in a study that included patients with CKD Stage 3-5 which showed that hyperuricemia is not risk factor for progression of CKD or need for renal replacement therapy.
In contrast, three studies on patients with IgA nephropathy found an association between hyperuricemia and progression of IgA.
Effect Of Intervention
Small studies have provided promising data supporting the use of uric acid-lowering drugs to retard the progression of CKD.
However, the exact mechanism by which these drugs achieve this effect is unclear.
In a study published in 2011 by the journal Hypertension, the authors, with Yan Miao as lead author, performed a post hoc analysis of the Reduction of Endpoints in Non-insulin-Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan Trial (RENAAL trial) to determine if the renoprotection derived from losartan is related to its urate-lowering effect.
This group concluded that one-fifth of losartan’s renoprotective effect could be attributed to its urate-reducing properties.
The authors, however, admitted that residual confounding factors might have been unaccounted for and that prospective randomized-controlled trials should be performed to confirm the results of the study.
In a randomized controlled trial published in 2010 by the Iranian Journal of Kidney Diseases, on the other hand, its authors, with Ali Momeni as lead author, performed a randomized trial on patients with diabetic nephropathy.
Momeni et al. reported a reduction in proteinuria in the allopurinol-treated group.
However, both the control and allopurinol-treated groups were maintained on ACE-inhibitors throughout the duration of the study. In addition, it is unclear how much of the proteinuria reduction can be attributed to allopurinol.
Furthermore, the sample size was small and when the authors measured the serum creatinine, there was no significant difference in both groups.
Both studies studied the use of allopurinol in diabetic population, making it difficult to generalize the results to the CKD population.
In a study published in 2010 by the Clinical Journal of the American Society of Nephrology, the authors, with Marian Goicoechea as lead author, addressed this issue by performing a prospective, randomized controlled trial on 113 patients with moderate CKD to determine the effect of allopurinol (100 mg/day) on CKD progression.
In this study, allopurinol treatment reduced serum uric acid level and C-reactive protein in the treatment group compared to the control group.
Blood pressure control was similar between the two treatment arms but the eGFR (measured by MDRD) worsened in the control group after 24 months.
The authors concluded that allopurinol treatment slowed down renal disease progression independent of:
- age
- gender
- diabetes
- C-reactive protein
- albuminuria
- use of renin-angiotensin blockers
Similar results were obtained from a prospective randomized trial, which included 54 patients with hyperuricemia and CKD 3-4 with proteinuria. It was published in 2006 by the American Journal of Kidney Diseases, with Yui-Pong Siu as lead author.
Although most of the studies mentioned above have encouraging results, they fail to establish the exact mechanism by which allopurinol or losartan can be beneficial for CKD.
In their study published in 2008 by The New England Journal of Medicine, Dr. Daniel I. Feig, Dr. Duk-Hee Kang, and Dr. Richard J. Johnson pointed out that some of the benefits derived from allopurinol such as improvement in endothelial function in patients with hyperuricemia and heart failureor diabetes were not noted in patients taking other uric-acid lowering medications.
Furthermore, most of the trials were small and involved highly specialized groups making the results difficult to generalize.
For these reasons, treatment of asymptomatic hyperuricemia is not currently recommended.
Conclusion
Over the past decade, there is mounting evidence that uric acid may play an important role in the development of:
- hypertension
- cardiovascular disease
- chronic kidney disease
Animal studies have provided useful information, and helped elucidate the mechanisms that underlie these associations.
Unfortunately, studies in humans investigating these associations have provided conflicting results.
At present, there is evidence that uric acid plays a role in the pathogenesis of hypertension, particularly in the young population with new-onset hypertension.
It is unclear why this relationship is not as evident in populations with established hypertension.
Some authors think that patients with longstanding hypertension may already have renal microvascular disease accounting for their hypertensive condition dampening the effect of uric acid in this population.
A similar trend is seen in studies looking into the role of uric acid and renal disease.
At most, we can say that uric acid may play a role in promoting chronic kidney disease among populations with normal renal function at baseline.
Still, its role in the progression of chronic kidney disease remains unclear.
Despite the multitude of studies conducted on uric acid over the past years, there is no compelling evidence to treat asymptomatic hyperuricemia for primary prevention of hypertension, cardiovascular disease, or renal disease.
Larger interventional clinical trials are needed to establish the role of uric acid in chronic kidney disease and other diseases.

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles