Fungicidal Assemblies And Their Mode Of Action
The major factors determining the activity of fungicidal assemblies are the mobility of the antimicrobial moiety, the charge on the fungus cell, and the size and shape of the antifungal assembly.
Author:Suleman ShahReviewer:Han JuFeb 19, 20241.7K Shares195.5K Views
Fungicidal assemblies can be built from lipids, polymers, and/or drugs to yield optimal activity against fungus in virtual absence of hemolysis/haemolysis.
Candida (Candida albicans) has often been used as a model for testing novel fungicidal assemblies both in vitro and in vivo.
Fungicidal drugs require appropriate formulations to improve their therapeutic index at low cost and toxicity.
Inexpensive synthetic lipids or surfactants and biocompatible, water soluble polymers can be assembled to provide novel vehicles for carrying the fungicidal drugs or eventually being the fungicidal agent themselves.
In this critical review, perspectives of some important fungicidal assemblies of low toxicity and cost are disclosed.
In addition, major factors such as mobility of the quaternary ammonium moiety, charge of the fungus cell, and the size and shape of the fungicidal nanostructures determining their mode of action are presented.
Preliminary Discussion
The development of novel fungicidal assemblies is important to circumvent the generally high toxicity of antifungal drugs and the problem of fungus resistance derived from the widespread use of such drugs in clinics.
Drugs of choice for treating fungal infections belong to different classes, such as:
- the azoles, which inhibit the synthesis of ergosterol
- the echinocandins, which inhibit the cell wall synthesis
- the polienic antibiotics(e.g., amphotericin B and nystatin), which combine with ergosterol in the fungus membrane to form pores thereby altering the membrane permeability
- the nucleoside analogues, which inhibit the synthesis of nucleic acids
- some antibiotics(e.g., griseofulvin), which inhibit cell division by hampering the synthesis of the microtubules
Alternative and less toxic antifungal formulations may include natural products, synthetic agents, and polymeric materials, such as some:
- alkaloids
- peptides
- saponins
- essential oils
- synthetic amphiphiles
- polymers with quaternary nitrogen atoms
- lipids or polymers loaded or not with antifungal compounds
Candida (Candida albicans) is the most important fungal opportunistic pathogen that can invade the bloodstream and disseminate to internal organs causing life-threatening invasive candidiasis.
The aim of this review was to discuss fungicidal assemblies and their mode of action.
Fungicidal Supramolecular Assemblies
The therapeutic index of toxic drugs can be considerably increased by appropriate formulation.
Among the classical fungicides, amphotericin B (AB) is a good example of this statement with several improved formulations available such as:
- nanoparticles
- liposomal AB
- AB colloidal dispersion
- AB lipid complex
- AB in microspheres
However, the formulation cost is an important issue mainly for neglected infectious diseases in developing countries (e.g., leishmaniasis).
In this regard, a low-cost AB lipid formulation using an inexpensive and synthetic cationic lipid (dioctadecyldimethylammonium bromide or DOD/AB) at low drug to lipid molar ratios was developed based on the high affinity of AB for the borders of cationic bilayer fragments (BFs).
Despite the relatively high dose of the pro-inflammatory and toxic cationic lipid, the DOD/AB formulation displayed:
- a high therapeutic index in vivo
- low nephrotoxicity
- low general toxicity
Based on the coating of AB aggregates with the DOD cationic bilayer, DOD concentrations could be reduced in AB/DOD formulations prepared at high drug to lipid molar ratios.
In order to improve the colloidal stability of AB/DOD or DOD/AB, the cationic assemblies were added with biocompatible consecutive layers of polyelectrolytes, such as:
a. the negatively charged carboxymethyl cellulose (CMC)
b. the positively charged poly(diallyldimethylammonium chloride) solution (PDADMAC) - also can be written as poly(diallyl dimethyl ammonium chloride)
When added, they yield robust fungicidal activity both at high and low drug to lipid molar ratios.
Later on, unloaded assemblies of DOD/CMC/PDDA and the PDDA polyelectrolyte itself were described as potent bactericides or fungicides in complete absence of any classical antifungal drug.
Among these assemblies with high fungicidal activity in vitro only the DOD/AB assembly was evaluated in vivo against systemic candidiasis in mice.
All other fungicidal assemblies still need to be tested in vivo.
- food processing
- mining industries
- paper manufacturing
- water treatment
Furthermore, this polyelectrolyte can be easily combined with other molecules (e.g., lipids and proteins) or their assemblies (e.g., bilayers, nanoparticles, and drugs both in the form of dispersions or nanostructured coatings) protecting different materials against fungus attack and biodegradation.
Among the azoles, miconazole (MCZ) was used as a model drug to obtain DOD/MCZ assemblies at low drug to lipid molar ratios based on MCZ solubilization in DOD bilayers.
At high drug to lipid molar ratios, MCZ cationic aggregates in water solution were covered by anionic sodium dihexadecyl phosphate(DHP) BF.
The minimal fungicidal concentrations (MFC) for MCZ in ethanol and in formulations with DOD BF (DOD/MCZ) or DHP BFs (DHP/MCZ) were determined against C. albicansATCC90028 yielding improved drug efficiency in comparison with Zoltec (from the manufacturer of carbon fiber in St. Louis, Missouri), the classical fluconazole formulation.
DOD BF-loading capacity relative to miconazole was determined from size distributions as 0.5 millimolar (mM). MCZ in 5 mM. DOD dispersed as DOD BF.
BF-loading capacity relative to amphotericin B was 0.1 mM. AB in 2 mM. DOD assembled as BF.
DOD by itself kills Cryptococcus neoformansand Candida sp.at 2 and 2 to greater than 250 mg/L of MFC, respectively.
Both AB/DOD and MCZ/DOD assemblies (drug particles surrounded by lipid) interacted with C. albicansfor 48 hours, yielding lower MFCs than those for the respective drug acting alone.
At high drug to lipid molar ratio, only the DOD-covered MCZ particles (MCZ/DOD) exhibited synergistic action against C. albicans.
It was observed that the lipid capsule retarded drug action but the formulation was very effective given enough time.
Fungicidal Activity Of The Quaternary Ammonium Moiety - Mode Of Action
The antifungal activity of agents bearing the quaternary ammonium moiety largely depends on molecular structure.
While substantial fungicidal activity was described for the micelle-forming quaternary ammonium surfactants, the bilayer forming, double-chained DOD lipid with long C18 hydrocarbon chains did not show the ability to move from the bilayer assembly to the fungus cell membrane.
There is a poor fungicidal activity of DOD BF or large vesicles (LV) against C. albicans.
Adsorption isotherms on C. albicansfor cetyltrimethylammonium bromide (CTAB), DOD from LV, and DOD from BF revealed the competitive adsorption of DOD LV onto C. albicanscells: increasing DOD concentration reduced DOD adsorption onto the cells due to the preferential vesicle-vesicle association instead of the vesicle-cell association.
In contrast to other neutral or anionic surfactants such as sodium dodecyl sulfate, CTAB did not cause the disruption of the fungus cell membrane as depicted from the absence of leakage of phosphorylated compounds after interaction of C. albicanswith CTAB over a range of concentrations.
Fungus death could be associated with a change of the cell surface charge from negative to positive as determined from measurements of the cell electrophoretic mobility.
In order to cause fungus death, the cationic quaternary ammonium moiety has to:
- absorb onto the cell
- change its charge
- penetrate through the cell wall reaching the fungus cell membrane
The rigid gel state of the DOD bilayer hampers its penetration into the fungus cell wall and cytoplasmic membrane explaining the poor fungicidal activity of the DOD cationic lipid in comparison with CTAB or other micelle-forming cationic surfactants.
Positively charged and highly fluid bilayers of dimyristoylphosphatidylcholine (DMPC)/CTAB over a range of CTAB molar ratios can incorporate chlorin e6 (Ce6) photosensitizer substantially improving its adsorption and uptake by C. albicans and facilitating the photodynamic inactivation of the fungus.
However, these liposomes containing CTAB were not able to kill C. albicans, suggesting the larger affinity of CTAB for the DMPC fluid bilayer than the one for the fungus membrane.
CTAB monomers coming from CTAB micelles, on the other hand, are excellent fungicides due to their high mobility in solution.
The quaternary ammonium moiety can only interact with the fungus cells if its host molecule is mobile.
A good example can be found in the dentistry field, where resins have been modified with pendant and permanent quaternary ammonium groups.
Grafting of a quaternary ammonium antimicrobial component in a polymer network can be achieved through copolymerization of the antimicrobial monomers with the conventional methacrylate monomers.
While free, non-polymerized quaternary ammonium monomers can rapidly kill oral pathogens, the antimicrobial component immobilized by polymerization does not exhibit equally strong inhibitory effects.
The weakened effects after polymerization are understandable from the restricted motility of the quaternary ammonium.
The high affinity between the biocompatible poly(methyl methacrylate) (PMMA) and CTAB or DOD allowed the preparation of antimicrobial films or antimicrobial particles with the quaternary ammonium compounds embedded in the polymeric network.
For these hybrid materials, CTAB mobility caused bacterial death both on bacteria contact and in solution, whereas DOD killed only on contact due to its low mobility in the polymer matrix and high affinity for the PMMA polymer.
Novel hybrid materials easily assembled from weak intermolecular interactions, such as:
| electrostatic attraction | π/π* stacking |
| hydrogen bonds | ion/ion interactions |
| van der Waals forces | ion/dipole interactions |
| hydrophobic effects | dipole/dipole interactions |
They can be very effective to build controllable nanostructures as films or nanoparticles.
Biocompatible polymers and their non-covalent assemblies with quaternary ammonium compounds deserve further investigation regarding their antifungal activity.
The mode of action of gemini surfactants (GS) bearing the quaternary ammonium moiety seems to involve lysis of the cell membrane and organelles with no apparent damage to the fungus cell wall.
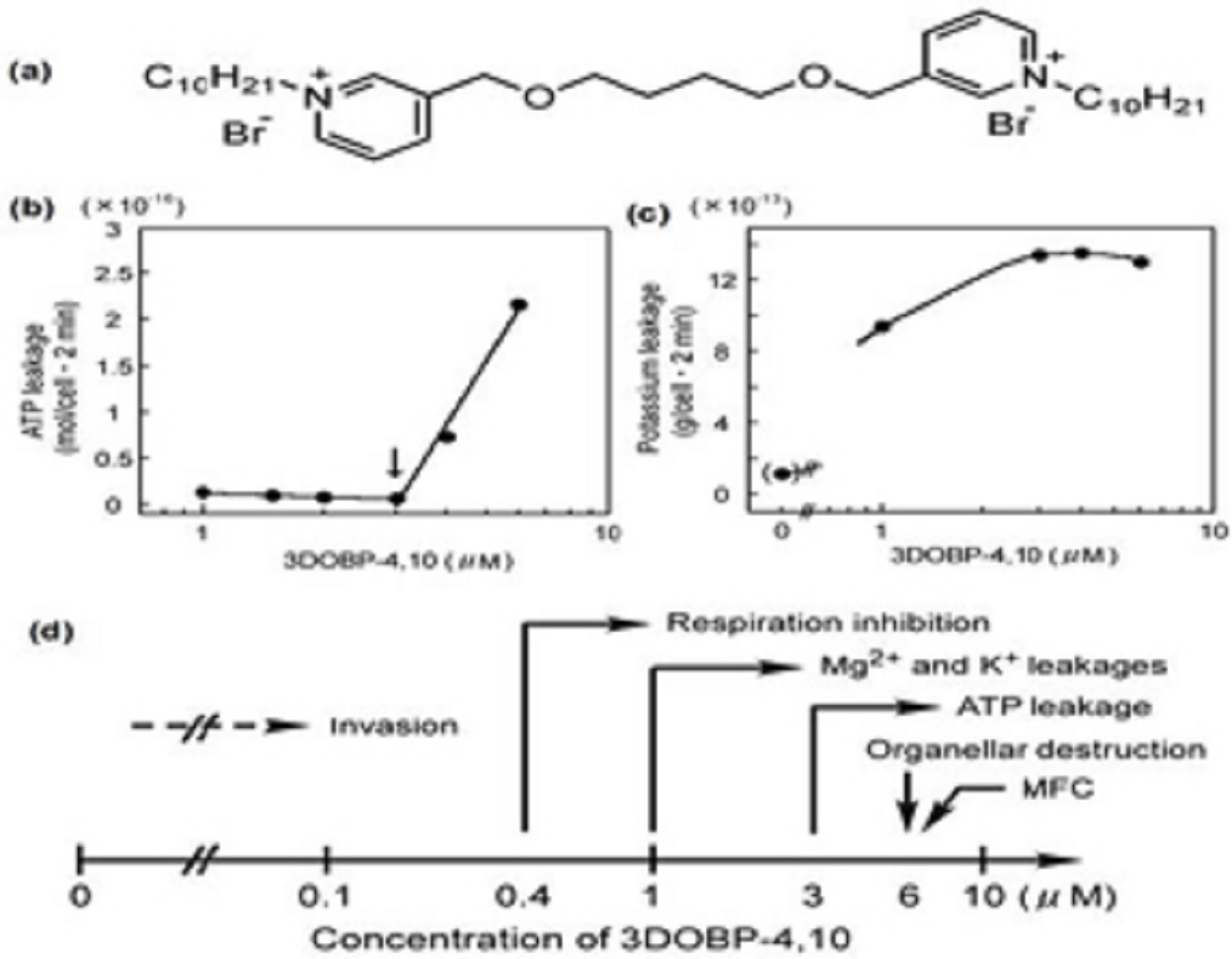
The gemini quaternary salt (gemini-QUAT) containing two pyridinium residues per molecule, 3,3ʹ-(2,7-dioxaoctane)bis (1-decylpyridiniumbromide) (3DOBP-4,10), exerts fungicidal activity against brewer’s yeast/baker’s yeast (Saccharomyces cerevisiae or S. cerevisiae) and causes respiration inhibition and the cytoplasmic leakage of ATP, magnesium, and potassium ions.
The gemini surfactant was more effective than the mono-QUAT N-cetylpyridinium chloride (CPC).
The production of reactive oxygen species was significantly elevated under aerobic conditions and associated with the activity of the gemini surfactant against S. cerevisiaeand C. albicans,with addition of scavengers of free radicals or anaerobic conditions reducing the fungicidal activity.
The evaluation of the antifungal activity of gemini quaternary ammonium salts over a range of hydrocarbon chain lengths showed that the compound with double 10 carbons chains was the most active one but exhibited significant toxicity against mammalian red blood cells around the minimal inhibitory concentrations (MIC) effective against the fungus.
Since C. albicansmay grow in different forms (e.g., yeast, pseudohyphal, and hyphal) and its virulence and biofilm formation is related to the switch from yeast to hyphae, inhibiting this switch hampers the biofilm formation.
Recently, C12 single and gemini cationic surfactants derived from arginine with spacers from 6-12 C were combined with a phospholipid (L-dilauroylphosphatidylcholine, DLPC) or cholesterol (CHOL)-forming cationic vesicles or aggregates with variable sizes for which the hemolytic activity and antimicrobial and antifungal activity were affected by the same parameters.
Their hemolytic activity is lower than one of the bis(QUATS) gemini surfactants as their antimicrobial activity.
For triblock polymers with the quaternary ammonium as the outermost block, simple changes to the core served to direct the self-assembly into distinct morphologies: spheres and rods.
Despite the vast majority of material consisting of poly(lactide) (interior block) and cationic polycarbonates (exterior block) testing the spherical and rod-like morphologies for antimicrobial properties showed that both possessed broad-spectrum activity (Gram-negative and Gram-positive bacteria as well as fungi) with minimal hemolysis, although only the rod-like assemblies were effective against C. albicans.
These triblock assemblies acted similarly to PDDA and its discoidal assemblies.
For the quaternary ammonium hybrid assemblies with water soluble polymers, optimal fungicidal and non-hemolytic activities have been achieved using the self-assembly of BFs, CMC and the PDDA antimicrobial polymer.
PDDA as a fungicidal agent has an MFC equal to 0.4 μg/mL and is effective in complete absence of hemolysis, contrasting with the poor activity of DOD against the fungus.
Furthermore, PDDA is easily assembled with proteins (e.g., albumin) to yield non-hemolytic nanoparticles.
Important assemblies described for AB were non-hemolytic cross-linked albumin microspheres (5 μ of mean diameter) or hybrid nanoparticles (0.08 μ of mean diameter), self-assembled from lipid and consecutive layers of water-soluble hydrophilic polymers, yielding effective AB formulations.
Conclusion
Fungicidal activity and low toxicity of antifungal assemblies requires the controllable self-assembly of toxic and biocompatible materials.
This critical review emphasizes how readily available materials including the antimicrobial quaternary ammonium moiety and water-soluble hydrophilic polymers can be assembled to yield non-hemolytic and efficient fungicidal nanostructures.
Based on the absence of hemolysis only, some promising combinations have been described that should be further tested for antifungal activity.
Based on the fungicidal activity only, some promising combinations have been described that should be further tested for hemolytic activity.
Among the most promising fungicidal agents is PDDA, which is basically a non-hemolytic, water-soluble hydrophilic polymer, with excellent antimicrobial activity easily assembled with proteins like albumin to yield non-hemolytic nanoparticles, although the hemolysis test was not yet performed.
Amphotericin B can be incorporated in cross-linked albumin microspheres (5 μ of mean diameter) or in hybrid nanoparticles (0.08 μ of mean diameter), self-assembled from lipid and consecutive layers of water-soluble hydrophilic polymers, yielding effective AB formulations.
PDDA itself or in nanostructured, hybrid lipid-polymer particles efficiently kills C. albicansin the absence of hemolysis.
The gemini assemblies seldom satisfy the low toxicity and high fungicidal activity requirements for use in vivo and need to be formulated with less toxic vehicles.
Further studies are recommended for fungicidal assemblies.

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles